Association Between Multifidus Intramyocellular Lipids and Pain-Related Disorders, Lumbar Spine Dysfunction, Gait Disturbance in Patients with Chronic Low Back Pain: A Cross-Sectional Study Using the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire
Izaya OGON1*, Hiroyuki TAKASHIMA2, Tomonori MORITA1, Mitsunori YOSHIMOTO1, Yasushi FUJITA3, Tsuneo TAKEBAYASHI4 and Atsushi TERAMOTO1
1Department of Orthopaedic Surgery, Sapporo Medical University School of Medicine, Sapporo, Japan
2Faculty of Health Sciences, Hokkaido University, Sapporo, Japan
3Department of Orthopaedic Surgery, Nishioka Daiichi Hospital, Sapporo, Japan
4Department of Orthopaedic Surgery, Sapporo Maruyama Orthopaedic Hospital, Sapporo, Japan
Abstract
Background and aims: Fat degeneration in the multifidus muscle (Mm) was more common than in other trunk muscles. We hypothesised that the lipid contents of the Mm and patient-reported quality of life (QOL) are related. However, there are no papers examining the association between fatty degeneration of the Mm and patient-reported QOL using magnetic resonance spectroscopy (MRS). This cross-sectional study aimed to investigate the association between patient-reported QOL, evaluated with the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ), and the intramyocellular lipid (IMCL) and extramyocellular lipid (EMCL) contents of the Mm and psoas major (PM) by MRS in patients with chronic low back pain (CLBP).
Materials and Methods:
Forty-eight patients (mean age, 64.2 ± 13.2 years; age range, 41–79 years) with nonspecific CLBP underwent MRS for the quantification of IMCL and EMCL of the right Mm and PM in a volume of interest at L4/L5. All subjects underwent MRS and completed the JOABPEQ in the same day. We performed multiple linear regression analysis of the IMCL and EMCL contents of the Mm and PM with the five domains of the JOABPEQ adjusted for age, sex, and body mass index.
Results: The IMCL content of the Mm was correlated with pain-related disorders (standardised partial regression coefficient (β)=−0.59, p<0.01), lumbar spine dysfunction (β=−0.64, p<0.01), and social life dysfunction (β=−0.31, p<0.01) which showed moderate negative correlation, but not with psychological disorders. The EMCL content of the Mm was not correlated with the five domains of the JOABPEQ. The IMCL and EMCL contents of the PM was not correlated with the five domains of the JOABPEQ.
Conclusions: IMCL content of the Mm was significantly correlated with the pain-related disorders, lumbar spine dysfunction, gait disturbance, and social life dysfunction domain scores of the JOABPEQ. Future studies using magnetic resonance spectroscopy of the Mm in patients with CLBP may help optimize exercise strategies using IMCL as an index to enhance patient-reported quality of life.
Introduction
Low back pain (LBP) is a serious chronic condition with a worldwide prevalence 1, 2, and the estimation is that approximately 23.0% of LBP will develop chronic low back pain (CLBP) 3. The 2010 Global Burden of Disease Study revealed that the LBP disability-adjusted life years increased from 58.2 million in 1990 to 83.0 million in 2010 4, although the majority of LBP patients experience non-specific symptoms that cannot be attributed to a serious disease what is called non-specific LBP 5. Despite this, the pathophysiology of LBP is poorly understood and there is inadequate correlation between investigative findings and clinical symptoms. LBP can be caused by various problems in any part of the interconnected network of spinal muscles, bones, discs, nerves, or tendons of the lumbar spine. Some mechanisms of LBP have been elucidated in previous studies 2, 6-19. Despite this, the pathophysiology of LBP is poorly understood and there is inadequate correlation between investigative findings and clinical symptoms.
Trunk muscles, such as the multifidus muscle (Mm) and psoas major (PM), are important in trunk function 20, 21. These muscles may be potential causes of LBP 22 and may be associated with LBP-induced disability. CLPB induces myoelectric activity and muscle remodelling, such as muscle atrophy, fatty degeneration, and altered fibre type 23-28. Magnetic resonance imaging (MRI)(29)29, ultrasonography 30, and computed tomography 31 are frequently used to assess fatty degeneration and muscle atrophy 32. At the early stage of paraspinal muscle degeneration, although fat replacement occurs in the paraspinal muscles, there is usually no significant change in the cross sectional area of the paraspinal muscles at this stage because of the space-occupying effect of fat 33. Therefore, fatty degeneration can sensitively reflect degeneration of the trunk muscles, and reduces the proportion of force-producing contractile tissue, thus affecting the function of the muscles in the lumbar spine 34.
Several investigators have recently reported quantitative evaluation of trunk muscle fatty degeneration using magnetic resonance spectroscopy (MRS) 7-9, 12, 15, 16, 18, 19. MRS analysis of muscle physiology has facilitated detailed analyses of muscular fat masses through the identification of intramyocellular lipids (IMCLs) and extramyocellular lipids (EMCLs), thereby enabling a detailed assessment of fatty degeneration 31, 35. IMCLs are undetectable when using conventional MRI as they appear as lipid droplets in skeletal muscle cells in close contact with skeletal mitochondria 36, 37. Moreover, IMCLs have been associated with aerobic metabolism 35, 38 and decrease after exercise 39. By contrast, EMCLs represent subcutaneous and extracellular fat in cells between muscle fibers 35, 40, 41.
Patient-reported quality of life (QOL) is an important component of CLBP; however, few studies have examined the association between patient-reported QOL and trunk muscle degeneration, as assessed using fat degeneration 26, 42. Teichtahl et al. 43 found that fat degeneration in the Mm was more common than in other trunk muscles. We hypothesised that the lipid contents of the Mm and patient-reported QOL are related. However, there are no papers examining the association between fatty degeneration of the Mm and patient-reported QOL using MRS. Therefore, this study aimed to investigate the association between patient-reported QOL, evaluated using the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ), and the IMCL and EMCL content of the Mm and PM, evaluated with MRS, in patients with chronic CLBP.
Methods
The study was conducted in accordance with the Declaration of Helsinki, after approval by the Ethics Committee of our institution (approval number: 23-131). All participants received written and verbal explanations of the study and provided informed consent before participation.
Participants
We included patients aged 41−79 years with nonspecific CLBP, characterized by pain, stiffness, and discomfort in the lower back from the twelfth rib to the lumbar or lumbosacral area where the source of the pain was difficult to identify. Furthermore, the symptoms of all participants persisted for more than 3 months despite conservative treatments such as medication and therapeutic exercise. The exclusion criteria included neoplasm, infection, fracture, or a history of lumbar vertebral surgery. We also excluded patients with neurological symptoms of the lower leg or obvious instability, which can be the source of LBP and improve with surgical treatment. All participants completed the JOABPEQ 44, which includes 25 questions corresponding to five domains: pain-related disorders, lumbar spine dysfunction, gait disturbance, social life dysfunction, and psychological disorders. The score of each domain was calculated, using the official guidelines, and ranged from 0 to 100 points in proportional to the patient’s clinical condition, with a higher score indicating a better health status. The test–retest reliability was confirmed by calculating the kappa and weighted kappa coefficients for each item. Both kappa and weighted kappa were more than 0.50 in all items, except in one item with 0.48. The lower 95% CI exceeded 0.4 in all items, except in two items with 0.39. This implied that the test–retest reliability of JOABPEQ was acceptable as a measurement of outcome 45. Superficial validity was checked in terms of the completion rate for filling out the questionnaire. Regarding the distribution of responses for each item, it was judged that none of the questions was too difficult to answer because the highest rate of nonrespose was 1.8%. With regard to deflection of an answer, the highest rate (78.3%) was concentrated on “yes” responses to question 1–14, although this was judged not to be inappropriate. Therefore, the distribution was not skewed, which would indicate “floor and ceiling” effects(46) 46.
MRI protocol and analysis of MRS data
This study used an MRI protocol and analysis methods for MRS data that have been described previously 7, 8, 9, 12, 15, 16, 18, 19. Briefly, we used a Signa HDx 1.5-T MRI System (GE Healthcare, Milwaukee, WI, USA) with a spine coil to obtain T2-weighted sagittal and transverse MRI images. From these images, the proton MRS volume of interest (VOI) was positioned at the center of the Mm and PM at L4/L5 on the right side (Fig. 1). The single-voxel point-resolved spectroscopy sequence was conducted with the following parameters: repetition time, 2000 ms; echo time, 35 ms; average number of signals, 64; VOI size, 15×15×15 mm (3.4 mL); and acquisition time, 164 s. Spectral data were used to measure the IMCL and EMCL contents using the LCModel software (Stephen Provencher, Inc., Oakville, ON, Canada). Data were transferred from the scanners to a Linux workstation. Metabolites were quantified using eddy current correction and water scaling. IMCL (1.3 ppm) and EMCL (1.5 ppm) methylene proton data were used for the statistical analyses. The assessments of IMCLs and EMCLs were scaled to an unsuppressed water peak (4.7 ppm) by the automatic function of the software and are expressed in institutional units. These data are displayed graphically, with the chemical shift on the x-axis and peak intensity on the y-axis, to enable metabolic identification (Fig. 2). All subjects underwent MRS and completed the JOABPEQ in the same day after a washout period of at least 4 weeks.
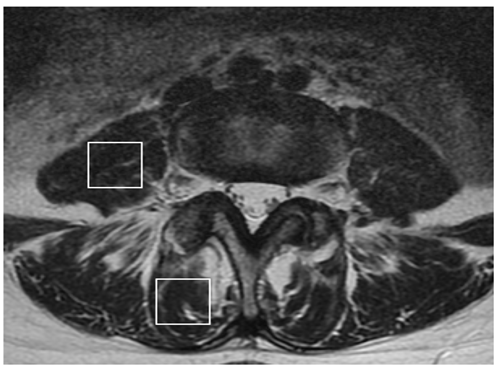
Figure 1: The volume of interest for magnetic resonance spectroscopy measurements of the right multifidus muscle (Mm) and psoas major (PM) indicated on the T2-weighted image at the L4/5 level.
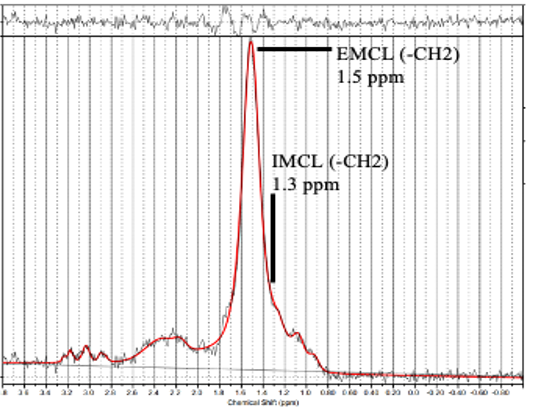
Figure 2: Proton magnetic resonance spectrum analyzed using LCModel software (Stephen Provencher, Inc., Oakville, ON, Canada). The following metabolites were identified: intramyocellular lipids (IMCL) (–CH2) methylene protons at 1.3 ppm; extramyocellular lipids (EMCL) (–CH2) methylene protons at 1.5 ppm.
Statistical analysis
Possible correlations of the IMCL and EMCL contents of the Mm and PM with the five domains of the JOABPEQ were analysed using Pearson’s correlation coefficient test. Significance was set at p < 0.05. Statistical analysis was performed using SPSS (version 27.0; IBM Corp., Armonk, NY, USA) by a MD researcher (I.O.). All numerical data are expressed as the mean ± standard deviation.
Results
Forty-eight patients (21 men and 27 women; mean age ± standard error of the mean, 64.2 ± 13.2 years; age range, 41–79 years) met the diagnostic criteria. The mean body mass index was 23.6 ± 2.1 kg/m2, and the mean visual analogue scale score was 54.3 ± 22.9 mm. The mean JOABPEQ scores were 43.7 ± 32.6, 53.9 ± 29.8, 52.6 ± 31.9, 46.0 ± 22.9, and 42.4 ± 17.3 for pain-related disorders, lumbar spine dysfunction, gait disturbance, social life dysfunction, and psychological disorders, respectively. The mean IMCL content of the Mm was 10.7 ± 11.1 (×102) mmol/L, and the mean EMCL content of the Mm was 5.5 ± 3.5 (×103) mmol/L. The mean IMCL content of the PM was 2.9 ± 3.5 (×102) mmol/L, and the mean EMCL content of the PM was 2.7 ± 2.8 (×103) mmol/L.
As shown in Table 1, the IMCL content of the Mm was correlated with pain-related disorders (standardised partial regression coefficient (β)=−0.59, p<0.01), lumbar spine dysfunction (β=−0.64, p<0.01), gait disturbance (β=−0.42, p<0.01), and social life dysfunction (β=−0.31, p<0.01) which showed moderate negative correlation, but not with psychological disorders. The EMCL content of the Mm was not correlated with the five domains of the JOABPEQ. As shown in Table 2, the IMCL and EMCL contents of the PM were not correlated with the five domains of the JOABPEQ.
Table 1: Multiple linear regression analysis of the IMCL and EMCL contents of the multifidus muscle with the five domains of the Japanese Orthopaedic Association Backpain Evaluation Questionnaire adjusted for age, sex, and body mass index.
|
Dependent variable |
Independent variable |
Regression coefficient |
Standard error |
Standardized partial regression coefficient |
p |
|
IMCL (mmol/L) |
Pain-related disorders |
−29.11 |
9.46 |
−0.59 |
<0.01 |
|
Lumbar spine dysfunction |
−38.31 |
7.95 |
−0.64 |
<0.01 |
|
|
Gait disturbance |
−20.42 |
11.35 |
−0.42 |
<0.01 |
|
|
Social life dysfunction |
−16.20 |
13.17 |
−0.31 |
<0.05 |
|
|
Psychological disorders |
−9.06 |
30.32 |
−0.04 |
0.80 |
|
|
EMCL (mmol/L) |
Pain-related disorders |
8.15 |
33.10 |
0.03 |
0.85 |
|
Lumbar spine dysfunction |
9.92 |
23.12 |
0.09 |
0.62 |
|
|
Gait disturbance |
9.10 |
30.23 |
0.04 |
0.79 |
|
|
Social life dysfunction |
−11.20 |
15.54 |
−0.14 |
0.33 |
|
|
Psychological disorders |
−10.10 |
18.43 |
−0.10 |
0.51 |
Table 2: Multiple linear regression analysis of the IMCL and EMCL contents of the psoas major with the five domains of the Japanese Orthopaedic Association Backpain Evaluation Questionnaire adjusted for age, sex, and body mass index.
|
Dependent variable |
Independent variable |
Regression coefficient |
Standard error |
Standardized partial regression coefficient |
p |
|
IMCL (mmol/L) |
Pain-related disorders |
9.02 |
30.42 |
0.04 |
0.79 |
|
Lumbar spine dysfunction |
7.12 |
34.15 |
0.02 |
0.91 |
|
|
Gait disturbance |
10.63 |
17.21 |
0.12 |
0.49 |
|
|
Social life dysfunction |
7.15 |
34.01 |
0.02 |
0.90 |
|
|
Psychological disorders |
9.55 |
29.11 |
0.05 |
0.76 |
|
|
EMCL (mmol/L) |
Pain-related disorders |
8.15 |
33.10 |
0.03 |
0.85 |
|
Lumbar spine dysfunction |
9.92 |
23.12 |
0.18 |
0.36 |
|
|
Gait disturbance |
9.25 |
35.02 |
0.16 |
0.39 |
|
|
Social life dysfunction |
9.90 |
23.21 |
0.09 |
0.63 |
Discussion
In this cross-sectional study, we analyzed the IMCL and EMCL content of the Mm and PM using MRS and identified their correlations with JOABPEQ scores. Several previous studies have reported an association between LBP and fat degeneration in trunk muscles using various indicators and imaging techniques and revealed an increase in fat degeneration 47-50. These studies used the fat fraction of the multipoint Dixon technique to assess degeneration, whereas studies using MRS comprehensively have evaluated degeneration with respect to overall fat content. In this study, we distinguished adipose tissue from IMCL and EMCL as fat content in the Mm and PM.
We found that the IMCL of the Mm was significantly correlated with the pain-related disorders, lumbar spine dysfunction, gait disturbance, and domains of the JOABPEQ scores. In a previous study, MRS analyses of the Mm in patients with CLBP revealed significantly higher IMCL levels than those of controls 7. The IMCL content reflects increased free fatty acid in the blood, free fatty acid uptake into skeletal muscle, and decreased free fatty acid oxidation capacity 36, 38. Therefore, IMCL may reflect the inflammatory state of skeletal muscles 35. An association between the IMCL content of the Mm and nociceptive pain (i.e., pain related to inflammation) has also previously been demonstrated 15. We speculated that the lack of association with psychological disorders may have involved other psychological factors, such as catastrophizing.
No associations were found between the IMCL and EMCL contents of the PM and the JOABPEQ scores. In a previous report 12, no association was identified between the IMCL and EMCL contents of the PM and LBP intensity. Arbanas et al. 51 also reported that no statistically significant differences of the fat infiltration in PM between controls and patients with CLBP were found. Among the trunk muscles, the PM has been described as having a clinically relevant function 22, 31. Future longitudinal studies using MRS and including more participants are warranted to analyse the relationship between PM parameters and CLBP disability.
Disc degeneration 52 and collapsed vertebral bodies 53 irreversibly develop with advancing age; however, an increase in the IMCL of the Mm may improve if conservative treatments, such as therapeutic exercise, are initiated. According to papers, modifications to the MF-IMCL could simplify management and treatment plans for arthrogenic muscle inhibition (AMI)-MF-induced CLBP 47, 54. Dual-echo and multi-echo MR imaging, which have demonstrated high agreement with MRS (R.0.87-0.92; P0.001), could also be used to determine this 47. As a result, MRS or other pertinent effective diagnostic procedures may be able to act as a proxy for MF-AMI-induced CLBP if IMCLs are present, and more especially if their level or percentage of existence varies. Moreover, IMCL levels or percentage-of-presence changes may function as a quantitative indicator or marker of the efficacy of any rehabilitation program or method if IMCLs can be used as a predictive marker for CLBP. The findings of this study indicate that the IMCL of the Mm is associated with higher disability levels in individuals with CLBP, and future research could enhance exercise strategies using the IMCL content as an index to enhance patient-reported QOL among patients with CLBP.
This study had some limitations. First, there was no control group. Second, this study was a cross-sectional study, and a longitudinal study design would have been beneficial.
Conclusion
This study investigated the relationship between LBP disability and the IMCL and EMCL content of the Mm and PM using MRS in patients with CLBP. The results indicated that the IMCL content of the Mm was significantly negatively correlated with the pain-related disorders, lumbar spine dysfunction gait disturbance, and social life dysfunction domains of the JOABPEQ scores. This finding suggests that MRS of the Mm in patients with CLBP may be beneficial in future research to enhance exercise strategies using the IMCL level as an index to enhance patient-reported QOL.
List of Abbreviations
CLBP, Chronic low back pain
JOABPEQ, Japanese Orthopaedic Association Back Pain Evaluation Questionnaire
LBP, low back pain
MRI, Magnetic resonance imaging
MRS, Magnetic resonance spectroscopy
PM, Psoas major
QOL, Quality of life
VOI, Volume of interest
Acknowledgements: We would like to thank Editage (www.editage.com) for English language editing.
Data Availability: The datasets generated and/or analyzed during the current study are not publicly available due to limitations of ethical approval involving the patient data and anonymity but are available from the corresponding author on reasonable request.
Declarations of Conflicts of Interest: None
Funding: None
References
- Deyo RA, and Weinstein JN. Low back pain. N Engl J Med 2001; 344: 363–370.
- Ogon I, Takebayashi T, Takashima H, et al. Analysis of chronic low back pain with magnetic resonance imaging T2 mapping of lumbar intervertebral disc. J Orthop Sci 2015; 20: 295–301.
- Ogon I, Takebayashi T, Takashima H, et al. Magnetic resonance spectroscopic analysis of multifidus muscles lipid content and association with spinopelvic malalignment in chronic low back pain. Br J Radiol 2017; 90: 20160753.
- Ogon I, Takebayashi T, Takashima H, et al. Quantitative analysis concerning atrophy and fat infiltration of multifidus muscle with magnetic resonance spectroscopy in chronic low back pain. Spine Surg Relat Res 2019; 3: 163–170.
- Ogon I, Takebayashi T, Takashima H, et al. Analysis of neuropathic pain using magnetic resonance imaging T2 mapping of intervertebral disc in chronic low back pain. Asian Spine J 2019; 13: 403–409.
- Ogon I, Takebayashi T, Takashima H, et al. Multifidus muscles lipid content is associated with intervertebral disc degeneration: a quantitative magnetic resonance imaging study. Asian Spine J 2019; 13: 601–607.
- Ogon I, Takashima H, Morita T, et al. Association between spinopelvic alignment and lumbar intervertebral disc degeneration quantified with magnetic resonance imaging T2 mapping in patients with chronic low back pain. Spine Surg Relat Res 2020; 4: 135–141.
- Ogon I, Takashima H, Morita T, et al. Relevance between Schmorl’s node and lumbar intervertebral disc degeneration quantified with magnetic resonance imaging T2 mapping in chronic low back pain. Asian Spine J 2020; 14: 621–628.
- Ogon I, Takebayashi T, Takashima H, et al. Imaging diagnosis for intervertebral disc. JOR Spine 2020; 3: e1066.
- Ogon I, Takashima H, Morita T, et al. Is the lipid content of the psoas major correlated with chronic low back pain and spinopelvic alignment? A magnetic resonance spectroscopic study. Asian Spine J 2020; 14: 430–437.
- Ogon I, Iba K, Takashima H, et al. Magnetic resonance spectroscopic analysis of multifidus muscles lipid contents and association with nociceptive pain in chronic low back pain. Asian Spine J 2021; 15: 441–446.
- Ogon I, Iba K, Takashima H, et al. Association between lumbar segmental mobility and intervertebral disc degeneration quantified by magnetic resonance imaging T2 mapping. N Am Spine Soc J 2021; 5: 100044.
- Ogon I, Teramoto A, Takashima H, et al. Factors associated with low back pain in patients with lumbar spinal stenosis: a cross-sectional study. BMC Musculoskelet Disord. 2022; 23: 552.
- Ogon I, Teramoto A, Takashima H, et al. Association between visceral fat chronic low back pain and central sensitization in patients with lumbar spinal stenosis. J Back Musculoskelet Rehabil 2022; 35: 1035-1042.
- Takashima H, Takebayashi T, Ogon I, et al. Evaluation of intramyocellular and extramyocellular lipids in the paraspinal muscle in patients with chronic low back pain using MR spectroscopy: preliminary results. Br J Radiol 2016; 89: 20160136.
- Takashima H, Takebayashi T, Ogon I, et al. Analysis of intra and extramyocellular lipids in the multifidus muscle in patients with chronic low back pain using MR spectroscopy. Br J Radiol 2018; 91: 20170536.
- Wilke HJ, Wolf S, Claes LE, et al. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study. Spine (Phila Pa 1976) 1995; 20: 192–198.
- Bogduk N, Pearcy M, Hadfield G. Anatomy and biomechanics of psoas major. Clin Biomechã1992; 7: 109–119.
- Ranger TA, Cicuttini FM, Jensen TS, et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J 2017; 17: 1729–1748.
- Cheung KM, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009; 34:934–940.
- Hebert JJ, Koppenhaver SL, Parent EC, et al. A systematic review of the reliability of rehabilitative ultrasound imaging for the quantitative assessment of the abdominal and lumbar trunk muscles. Spine (Phila Pa 1976) 2009; 34: E848–E856.
- Danneels LA, Vanderstraeten GG, Cambier DC, et al. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J 2000; 9: 266–272.
- Boesch C, Machann J, Vermathen P, et al. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed 2006; 19: 968–988.
- Boesch C. Musculoskeletal spectroscopy. J Magn Reson Imaging 2007; 25: 321–338.
- Boesch C, Kreis R. Observation of intramyocellular lipids by 1H-magnetic resonance spectroscopy. Ann N Y Acad Sci 2000; 904: 25–31.
- Schrauwen-Hinderling VB, Hesselink MK, Schrauwen P, et al. Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring) 2006; 14: 357–367.
- White LJ, Ferguson MA, McCoy SC, et al. Intramyocellular lipid changes in men and women during aerobic exercise: a (1) H-magnetic resonance spectroscopy study. J Clin Endocrinol Metab 2003; 88: 5638–5643.
- Srikanthan P, Singhal A, Lee CC, et al. Characterization of intra-myocellular lipids using 2D localized correlated spectroscopy and abdominal fat using MRI in type 2 diabetes. Magn Reson Insights 2012; 5: 29–36.
- Velan SS, Said N, Durst C, et al. Distinct patterns of fat metabolism in skeletal muscle of normal-weight, overweight, and obese humans. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1060–R1065.
- Hicks GE, Simonsick EM, Harris TB, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci 2005; 60: 1420–1424.
- Ranger TA, Cicuttini FM, Jensen TS, et al. Paraspinal muscle cross-sectional area predicts low back disability but not pain intensity. Spine J 2019; 19: 862-868.
- Fukui M, Chiba K, Kawakami M, et al. JOA back pain evaluation questionnaire (JOABPEQ)/JOA cervical myelopathy evaluation questionnaire (JOACMEQ). The report on the development of revised versions. April 16, 2007. The Subcommittee of the Clinical Outcome Committee of the Japanese Orthopaedic Association on low back pain and cervical myelopathy evaluation. J Orthop Sci 2009; 14: 348–65.
- Fischer MA, Nanz D, Shimakawa A, et al. Quantification of muscle fat in patients with low back pain: comparison of multi-echo MR imaging with single-voxel MR spectroscopy. Radiology 2013; 266: 555–563.
- Yanik B, Keyik B, Conkbayir I. Fatty degeneration of multifidus muscle in patients with chronic low back pain and in asymptomatic volunteers: quantification with chemical shift magnetic resonance imaging. Skeletal Radiol 2013; 42: 771–778.
- Wan Q, Lin C, Li X, et al. MRI assessment of paraspinal muscles in patients with acute and chronic unilateral low back pain. Br J Radiol 2015; 88: 20140546.
- D’Hooge R, Cagnie B, Crombez G, et al. Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Man Ther 2012; 17: 584–588.
- Haefeli M, Kalberer F, Saegesser D, et al. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine (Phila Pa 1976) 2006; 31:1522–1531.
- Chrischilles EA, Butler CD, Davis CS, et al. A model of lifetime osteoporosis impact. Arch Intern Med 1991; 151: 2026–2032.
