Rehabilitation of balance control with the rotatory chair protocol depends on rehabilitation onset and postural task difficulty in unilateral vestibular hypofunction patients
Michel Lacour1,4*, Laurent Tardivet2, Alain Thiry3
1Neurosciences Department, Aix-Marseille University/CNRS, Marseille, France
2Otorhinolaryngology Department, CHU Nice, 30 Voie Romaine, 06000 Nice, France
3Physiotherapist, 29 Bd Dubouchage, 06000 Nice, France
421 Impasse des Vertus - 13710 Fuveau (France)
Abstract
Purpose: Postural instability is a disabling symptom in patients with acute unilateral vestibular hypofunction (UVH). Vestibular rehabilitation (VR) with the unidirectional rotation paradigm has been shown recently to improve gaze stabilization in UVH patients, particularly when performed early after onset of the vestibular pathology, but its role on posture recovery remains unknown until today.
Methods: Effects on posture and balance recovery of early versus delayed VR with the rotatory chair protocol were analyzed under static and dynamic postural tasks performed in different visual conditions (eye open: EO, eyes closed: EC, optokinetic stimulation). Posture control was investigated through non-linear analyses of the stabilogram in three groups of patients submitted to the same VR program performed at different time periods after onset of the acute vertigo attack (early VR: first two weeks; late 1 VR: third and fourth weeks; late 2 VR: one month and more). The Dizziness Handicap Inventory (DHI) score was evaluated before and after VR.
Results: All the postural parameters (Postural Instability Index: PII, Spectral Power Density: SPD, Critical Point amplitude: CP amp, and Hausdorff Frequency: HF) were significantly modified in the UVH patients tested before VR compared to the controls. Greater instability (increased PII) associated with higher energy to control posture (enhanced SPD), higher CoP displacements without feedback corrections (increased CP amp), and lower time of automatic control of posture (decreased HF) was the typical pattern of the UVH patients. After rehabilitation and in static posturography conditions, all the postural parameters were improved in the three groups of patients, whatever the visual condition, without significant differences between the groups. By contrast, recovery of balance in the dynamic postural conditions was better only when rehabilitation was performed early. A lower percentage of fallers was observed in the early and late 1 group in the most challenging conditions with EC and optokinetic stimulation. In addition, the early group was the only one to show significant improvement of the postural parameters (PII, SPD, CP amp and HF), and the late 2 group the only one to show no significant changes. The late 1 group exhibited an intermediate recovery pattern. The DHI scores were significantly reduced in the early and late 1 groups only.
Conclusions: Posture control is strongly impaired in the UVH patients who display greater instability, higher body sway without feedback correction, and spend much more energy to keep balance. Postural recovery after VR does not depend on the time period between onset of pathology and beginning of VR when patients were tested in the easy postural tasks on a stable support. However, in the most challenging conditions on unstable support, without vision or moving visual environment, earlier the rehabilitation with the rotatory chair protocol, better the recovery. This latter result suggests a critical period to recover optimally the dynamic vestibulo-spinal function, similar to the early opportunity time window we have highlighted for the recovery of the dynamic vestibulo-ocular reflex.
Introduction
Posture control is based on the central integration of multisensory inputs arising from allocentric (vision), egocentric (somatosensory) and geocentric (vestibular) reference frames, and on an internal model of body position in space continuously updated by this sensory feedback to adapt the motor command to the environmental constraints1,2 or pathological conditions3. Regulation of body position in space for orientation and stabilization is automatically done at both subcortical and spinal levels for low challenging postural tasks like quiet standing, a motor-balance skill of everyday life4. In healthy subjects with stable support surface and fixed visual environment, the allocentric and egocentric sensory feedback is dominant compared to the geocentric input from the vestibular system5, which mainly contributes to activate the antigravitive muscles tonically, through the lateral and medial vestibulospinal pathways6. By contrast, under much more challenging conditions, the vestibular contribution to posture and balance control becomes predominant. Dynamic posturography findings in healthy subjects indicate that the sensory cues weighting for body sway stabilization relies mainly on vestibular inputs under sway referenced visual or somatosensory contexts7,8.
The tonic vestibular contribution to posture control has been demonstrated in both animal models of vestibular loss9 and vestibular patients10,11. Clinical studies showed that patients with acute unilateral vestibular loss had ipsilateral roll and frontal head tilt12, abnormal body alignment13, increased body sway in eyes open and eyes closed conditions compared to healthy controls10,14-16. The dynamic vestibular contribution to balance function was evidenced in such patients by modifications of the locomotor pattern, disequilibrium and falls to the lesion side17, and poor postural performances in dynamic posturography conditions when vision or somatosensory inputs were sway referenced7,8,11.
As a rule, the static postural deficits are compensated over weeks and months in a process referred to vestibular compensation in the literature, while the dynamic balance function remains poorly recovered in the most challenging conditions18,19. Sensory substitution mechanisms based on visual and somatosensory inputs are generally involved in the postural deficits compensation16,20-22, and it is fully accepted now that vestibular rehabilitation therapy (VR) is effective for improving balance, dizziness and quality of life in vestibular loss patients23-25. Not only VR is a safe and effective therapy that accelerates the recovery process, but it also optimizes the final level of vestibular compensation26.
One important point is to know whether there is a critical or sensitive period during the time course of recovery, a burning question underlined by the American Physical Therapy Association27 regarding the quality of life for the patients and the health-care costs for the society. Our investigations in vestibular-lesioned animal model based on early versus delayed sensorimotor activity after onset of the lesion provided the first demonstration that early is better28,29. However, this question is still under debate for vestibular patients30 since some clinical studies showed VR benefits when performed in the acute stage31,32 while others pointed to benefits at all phases33,34. Our paper on recovery of the dynamic visual acuity in UVH patients has provided for the first time direct comparisons of early and delayed VR, and it was clearly showed that earlier VR, better the recovery35.
Can this conclusion be extended to the recovery of posture and balance disorders? That is the topic of this study in which we have compared early and delayed VR in UVH patients submitted to the rotatory chair protocol. Used as a rehabilitation method for UVH patients as early as the end of the XXth century by French physiotherapists, the rotatory chair protocol consists in unidirectional rotations of the patient’s whole body towards the lesion side to reduce the response from the intact labyrinth and, therefore, to decrease the vestibular asymmetry seen acutely after the unilateral vestibular loss. Ushio et al.36 in unilateral labyrinthectomized macaques, then Sadeghi et al.37 in patients with chronic vestibular dysfunction, underlined the potential role of unidirectional rotations to rebalance the vestibular system. We have confirmed this statement in UVH patients rehabilitated with two different unilateral rotation paradigms38. The present study was aimed at examining early versus delayed VR interventions with the rotatory chair protocol in UVH patients. The outcomes were objective measurements of posture and balance control by static and dynamic posturography, and subjective evaluation with the dizziness handicap inventory score.
MATERIAL AND METHODS
Participants
This prospective study included 40 patients with UVH who were diagnosed as acute unilateral vestibulopathy (vestibular neuritis) on the basis of patients’ history and clinical examination. The criteria defined by Strupp and Magnusson39 were used for patients’ inclusion (acute onset of spinning vertigo, postural imbalance, nausea, spontaneous horizontal rotatory nystagmus, positive Head Impulse Test (HIT) to the disease side. The HIT was defined as pathological when the aVOR gain was below 0.65 and when overt and/or covert saccades were recorded. All patients underwent passive HIT and aVOR gain measurement using the VHIT Ulmer recording device (Synapsis, Marseille, France) to measure the deficit of the horizontal aVOR before VR. All patients showed pathological aVOR responses on the hypofunction side, while normal responses were recorded on the healthy side (Table I). Among the 40 patients, 31 had pathological HIT responses to horizontal canal test, vertical anterior canal test and posterior canal test on the hypofunction side, attesting of complete impairment of the superior and inferior branches of the vestibular nerve. The remaining 9 patients had impairment of the superior branch only with pathological HIT tests for both the horizontal canal test and vertical anterior canal test. The caloric test was not systematically performed because of its unpleasant side for patients, and when it was done the response was lacking on the lesion side. The VEMPs were not done due to lack of necessary equipment. Central vestibular or ocular motor dysfunctions as well as positional vertigo constituted exclusion criteria.
The whole population of UVH patients was subdivided into three groups on the basis of the moment when VR was started after onset of the vertigo attack (Table I). A first group (N = 19) was made of seven males and twelve females (mean age 59.3 ± 14.9 years; range 32–80 years; 15 with pathological impairment of both vestibular nerve branches) submitted to an early VR starting within the first two weeks after onset (mean 7.2 days: Fig. 1A-B). A second group included thirteen patients (6 males and 7 females; mean age 65.9 ± 15.5 years; range 36–82 years; 10 with both vestibular nerve branches impaired) receiving VR between the 3rd and the 4th week after onset (mean 22.9 days). The third group was composed of eight patients (2 males and 6 females; mean age 61.2 ± 13.8 years; range 34–79 years; 6 with both vestibular nerve branches impaired) who were tested in the time period 1–2 months (mean 44.1 days) after onset of the acute vertigo attack. It must be mentioned that, on the average, the patients of this third group had their first inclusion visit and their first VR session at time periods when patients of the early group had already finished their VR. The third group tested just before VR can be assimilated to a control group without specific rehabilitation and used to assess the potential effects of VR therapy by comparison with the early group after VR (see Fig. 1C). The three groups of patients were investigated for posture control (static posturography), balance performance (dynamic posturography), and subjective evaluation of their handicap (DHI score) before and after vestibular rehabilitation with the rotatory chair protocol (Figure 1A).
Table I: Characteristics of the three groups of unilateral vestibular hypofunction (UVH) patientsThe table shows the total number of UVH patients (N) and the number of patients in each of the three groups (n) submitted to early or later rehabilitation with the rotatory chair protocol. Gender, mean age (and range, in days), mean angular horizontal vestibulo-ocular reflex gain (aHVOR ± SD) on both the ipsilateral hypofunction and contralateral healthy sides, and mean time (and range, in days) between onset of the pathology and beginning of the vestibular rehabilitation are indicated.
| EARLY UR REHAB | LATE 1 UR REHAB | LATE 2 UR REHAB | ||
|---|---|---|---|---|
| N = 40 | n = 19 | n = 13 | n = 8 | |
| Gender | 7 Males 12 Females |
6 Males 7 Females |
2 Males 6 Females |
|
| Mean Age (Range) | 59.3 (32 – 80) |
65.9 (36 – 86) |
61.2 (34 – 79) |
|
| aHVOR Gain | Ipsilat | 0.18 ± 0.14 | 0.19 ± 0.12 | 0.14 ± 0.11 |
| Contral | 0.85 ± 0.08 | 0.88 ± 0.05 | 0.81 ± 0.09 | |
| Time from Onset (Mean and Range, days) | 7.2 (2 - 12) |
22.9 (16 - 30) |
44.1 (32 – 65) |
|
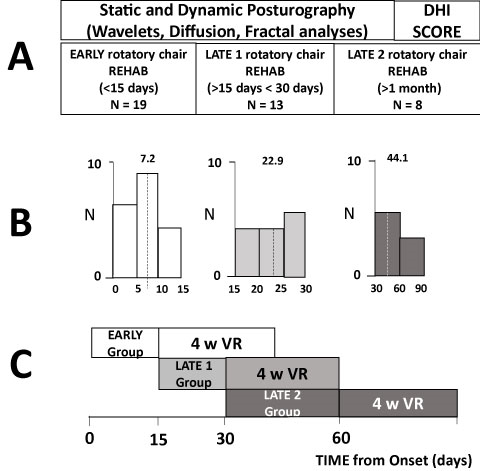
Figure 1A-C: Methods and Experimental Protocol
A) Illustration of the different non-linear analyses of the CoP displacements (wavelet transform, diffusion and fractal analyses) and questionnaire (Dizziness Handicap Inventory: DHI) used to investigate posture and balance functions in the static and dynamic posturography tests for the three groups of unilateral vestibular hypofunction (UVH) patients submitted to early and later rehabilitation with the rotatory chair protocol. The early rehabilitation (N=19) was made of patients rehabilitated in the first two weeks after onset of vestibular disesase, the late 1 group (N=13) between the third and the fourth week, and the late 2 group (N=8) after one month or more. B) Histograms showing the distribution of the patients in each group and the mean time delay between vertigo onset and beginning of the rehabilitation. C) Schematic drawing illustrating when the rehabilitation sessions (two times a week for four weeks) were provided to each group of UVH patients compared to onset of vestibular pathology.
Drug treatment was an exclusion criterion and patients were advised not to take anti-vertigo drug treatments after inclusion. Written informed consent to participate was obtained for each patient, the investigation was performed according to the Helsinki Declaration, and an ethic local committee (CCPPRB) approved the protocol.
Static and Dynamic Posturography
Experimental Setup
Posturography test consisted of recording the Center of foot Pressure (CoP) displacements during sequences of 30 s using a force platform (Multitest Equilibre, Framiral, Grasse, France: sampling frequency: 50 Hz) in patients standing quietly without voluntary movements of head and body. Six recording sessions composed the posturography test, which duration was 6 to 8 minutes. 15-30 second rest periods were given to the patients between each session. The first three sessions were performed with the patient standing on a stable support (static posturography), with eyes open (EO), then with eyes closed (EC), and thereafter with vision of a moving random visual pattern provided by an optokinetic device (Opto: Framiral, Grasse, France). The last three sessions were performed in the same experimental conditions (EO, EC, Opto) but with patients on unstable support (dynamic posturography). The platform was totally free to move in the 3D space, the patient’s own instability creating the displacements of the platform. Body sway was evaluated in each visual condition by computing the CoP over time.
Data Processing
The CoP displacements computed in the antero-posterior and medio-lateral directions were used to measure the static and dynamic postural performance of the patients. Non-linear analyses of CoP displacements were performed in order to accurately evaluate posture and balance control40. It consisted of applying the wavelet transform, the stabilogram diffusion analysis, and the fractional Brownian-motion analysis to the stabilograms (PosturoPro software, Framiral, Grasse, France).
The wavelet analysis has been described in detail in previous papers41-43. Briefly, this method is particularly appropriate to study non stationary signals like CoP displacements, and it provides a three-dimensional time frequency chart of body sway. The spectral power density (SPD: decimal logarithm scale visualized as a color code) gives the energy cost to maintain balance, and the Postural Instability Index (PII) provides a global score of posture control (Figure 2A-B). Higher the PII, higher the instability, and higher the SPD, higher the energy spent to control posture.
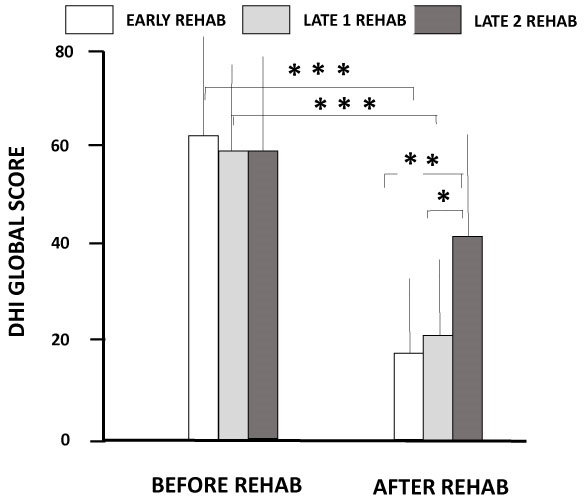
Figure 2: Dizziness Handicap Inventory scores before and after vestibular rehabilitation with the rotatory chair protocol
The global DHI scores incorporating the physical, functional and emotional items are shown (ordinates) for the three groups of patients before and after receiving the rehabilitation early after onset of vertigo attack (open histograms), or at later stages (late1 group: grey histograms; late 2 group: filled histograms). Significant differences between the pre- and post-rehabilitation DHI scores are shown with asterisks. *: p<0.05; **: p<0.01; ***: p<0.001.
The CoP trajectories were also studied with the stabilogram diffusion analysis44. The CoP trajectory analysis computed the square of the displacement between all pairs of points separated by a specified time interval Δt, then averaged over the number of Δt of the recording session, and repeated for increasing values of Δt. The analysis provides a unique planar stabilogram-diffusion plot defining a critical point which spatio-temporal coordinates approximate the region over which posture control switches from open-loop to close-loop control mechanisms. The diffusion analysis is particularly relevant to extract parameters from the raw posturography data directly related to the steady-state behavior of posture control or to the functional interactions with the neuromuscular mechanisms involved in the maintenance of upright stance. The amplitude of the critical point (CP amp, in mm2) estimates when feedback mechanisms intervene to avoid fall (higher the CP amplitude, higher the risk of fall).
The fractal analysis, based on statistical-mechanics45, is another way to estimate posture stability. It consists of determining if the CoP displacements are correlated, that is, linked by a causal relationship (CoP moving forward because previous backward displacement: feedback correction; close-loop control mechanism), or not correlated (random CoP trajectory, stochastic process: open-loop control mechanism). The number of Hausdorff points in each stabilogram, not correlated with each other, and their mean frequency were evaluated in order to evaluate the mean time-interval during which the patient remains stable without postural correction. In healthy non anxious subjects, higher the HF, more frequently the subject operates in open-loop, automatic control of posture.
Rotatory chair protocol
The rotatory chair protocol is a unidirectional rotation paradigm aimed at rebalancing the vestibular system. The patients were sitting in a rotatory chair (Framiral, Grasse, France) with eyes closed, the head tilted by 30° down to have the horizontal canal plan close to the horizontal. They were instructed to keep the eyes closed during the whole rotation of the chair and to open the eyes as soon as the chair rotation was stopped. At this moment, they were asked to fixate a visual target located 1.5 m in front of them, at eye level. Whole body rotations of the UVH patients were always performed toward the hypofunction side. They consisted of sudden high velocity rotation of the chair (200°/s-250°/s; acceleration: 1000°/s2) during three full 360° turns or more, depending on patient’s tolerance to the protocol, followed by a sudden stop of the chair. The time during which the patient reported the visual target was moving, as a result of the post-rotatory nystagmus, was measured after each chair rotation, and five to ten trials were successively done during the same session. As a rule, the time for extinction of the illusory target motion progressively reduces with repetition of the chair rotations due to the progressive decline in the post-rotatory response. Habituation of the intact labyrinth to the repetition of the rotations to the lesion side can be observed at the end of the session and retention at the beginning of the following session, an objective sign of the reduction of the vestibular imbalance (paper in preparation). The UVH patients in each of the three groups were submitted to the same training protocol, including an equal number of training sessions. These rehabilitation sessions were performed two times a week for four weeks after inclusion of the patients (Figure 1C).
Dizziness Handicap Inventory (DHI)
Each UVH patient was required to fill out the Dizziness Handicap Inventory questionnaire46. It consists of 25 items incorporating physical, emotional and functional aspects of vertigo and dizziness. Each item is evaluated on a three point scale: “yes”, “sometimes” or “no”, scored as 4, 2 or 0, respectively. The maximal scores were 28, 36 and 36 for the physical, functional and emotional items, respectively. A global score was obtained by adding all items, with a maximum of 100 points. The total DHI score was calculated before and just after the end of rehabilitation with the rotatory chair protocol.
Data analysis
Statistical analysis was carried out using analyses of variance (ANOVAs) with repeated measures. Groups (early, late 1, late 2), postural parameters (PII, SPD, CP amp, HF), and DHI score were the between-patients factors, and pre- and post-rehab sessions as the within-patients factors. Global evaluation was done with the Bonferroni’s multi-comparisons test. Supplementary ANOVAs were performed in order to test the influence of sources of variation (age, gender). To measure the effects of visual condition (EO, EC, Opto) on posture control in both the stable and unstable platform conditions, the four postural parameters (PII, SPD, CP amp, HF) were analyzed using a 4-way ANOVA. Post-hoc analysis was done with the Bonferroni’s multi-comparisons test. The probability level p < 0.05 was used to evaluate significant differences. It was verified that non-parametric tests (Mann-Whitney U test and Wilcoxon signed ranked test) more adapted to small size samples provided similar results.
The postural parameters recorded in the UVN patients in the static posturography test (EO, EC and Opto on stable support) were compared to the normative values (mean values ± SD) collected in a population of 225 healthy subjects in the same range of age (40-80 years). Moreover, the UVH patients tested in the most challenging conditions (EC and optokinetic stimulation on unstable support) failed generally to keep balance before rehabilitation, contrary to healthy subjects. The percentage of fallers in these two conditions was therefore evaluated before and after rehabilitation as a functional parameter to assess the effects of VR with the rotatory chair protocol.
RESULTS
The general ANOVA showed no significant differences for age and gender between the three groups of UVN patients. Significant differences were observed between the healthy controls and the three groups of UVH patients for all the postural parameters recorded before rehabilitation. Significant differences were also found between the pre-rehab and post-rehab for the postural performance and the DHI score.
DHI Score
The total DHI score incorporating the physical, functional and emotional items did not differ significantly between the three groups of UVH patients at the first visit before rehabilitation (p=0.68, 0.78 and 0.54 for the early, late 1 and late 2, respectively). The patients were in the same range of moderate handicaps (DHI = 60.9 ± 21.0, 59.1 ± 19.2, and 58.0 ± 22.5 for the early, late 1 and late 2 groups, respectively). The ANOVA with repeated measures between the pre- and post-rehabilitation values showed significant differences between the groups [F (1,37) = 104.4; p<0.0001]. After rehabilitation, the early and late 1 groups showed significant reductions of the global DHI score (21.2 ± 19.3 and 21.3 ± 23.6, respectively; p<0.0001), shifting from moderate to slight handicaps, with mean improvements of 39.7 points and 37.8 points, respectively. A non-significant reduction was observed in the late 2 group (40.7 ± 19.5; p=0.07) that still exhibited a moderate handicap with a mean reduction of 17.3 points only (Fig. 2). The early and late 1 groups differed significantly with the late 2 group (p<0.01 and p<0.05, respectively).
Posturography Data
All the UVH patients were able to keep balance without falling in the easiest static posturography tasks, that is, on the stable platform with EO, EC and Optokinetic stimulation. By contrast, most of them failed to keep balance in the most challenging conditions on the unstable platform. Figure 3A illustrates the raw wavelet plots of a typical UVH patient of the early group tested before rehabilitation. The 3D maps show the body sway frequency and the spectral power density as a color code in the frequency domain as a function of the recording time. Keeping balance is possible on stable support (upper graphs) while fall occurs without vision (EC) and with a moving visual surround (optokinetic: lower graphs). Figure 3B shows the improvement of the postural performance of this patient after rehabilitation, who keeps balance in all six conditions of the test.
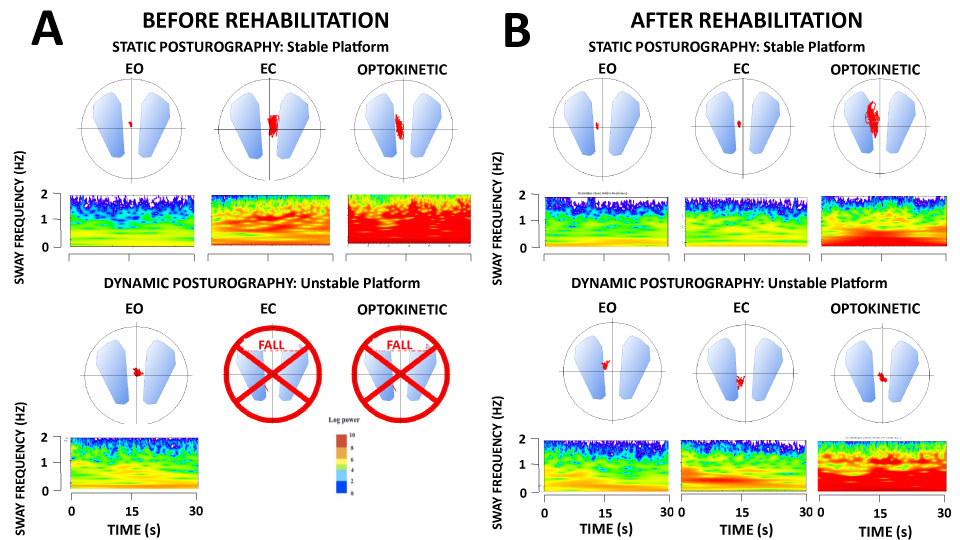
Figure 3A-B: Wavelet transform applied to the stabilogram of one representative unilateral vestibular hypofunction patient (UVH) of the early rehabilitation group examined with static and dynamic posturography
The wavelet transform, here applied to the CoP displacements in the antero-posterior direction provides a 3D chart of body sway with time on the abscissae (in seconds), the frequency content of the stabilogram on the ordinates (body sway in Hz), and the spectral power density shown with a color code (expressed in decimal Log). The figure shows the 3D maps recorded before (A) and after (B) rehabilitation of the patient in the three visual conditions (eyes open: EO; eyes closed: EC, and with optokinetic stimulation) on the stable platform (static posturography: upper plots) and the unstable platform (dynamic posturography: lower plots). Note the increased energy spent by this patient to control quiet standing before rehabilitation under EC and optokinetic visual conditions (hot colors in the low body sway frequency range), and his incapacity to keep balance in the most challenging dynamic posturography tests in the same visual conditions. Posture and balance functions were significantly improved in this patient after rehabilitation.
Static Posturography
The general ANOVA did not show significant differences between the 3 groups of patients examined before rehabilitation (F (2, 222) = 405.3; p=0.39), but a significant interaction group-visual condition (EO, EC, Opto) was found (F (2, 222) = 51.6; p<0.001). On the other hand, all the postural parameters recorded in the patients before rehabilitation differed significantly compared to those of the healthy controls. The figure 4 is the quantification of the PII recorded in the three visual conditions for each group of UVH patients. This global postural stability score derived from the wavelet transform is illustrated by boxplots showing the mean value with the first and third quartiles, and whiskers indicating the minimum and maximal PII values. Compared to the normative data from age-matched healthy controls represented by the blue colored area (mean ± SD), the mean PPI values recorded in the patients before rehabilitation were significantly higher with EO (p<0.001), with EC (p<0.001) and with optokinetic stimulation (p<0.001). After rehabilitation, the PII scores were close to the controls.
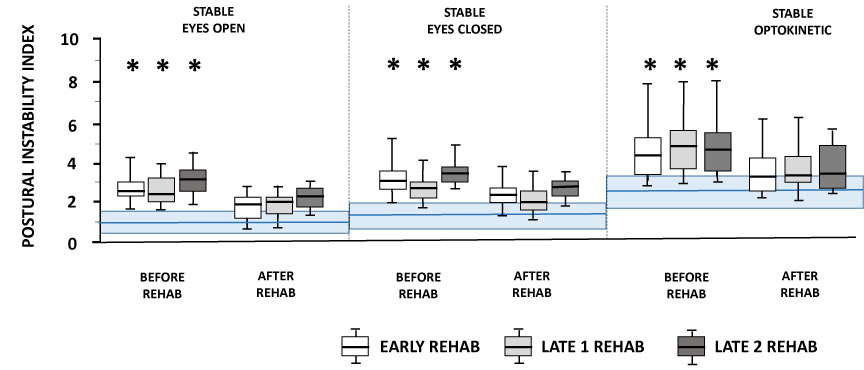
Figure 4: Changes in the Postural Instability Index after rehabilitation in the three groups of unilateral vestibular hypofunction (UVH) patients tested in static posturography conditions
The figure shows the Postural Instability Index score (ordinates) recorded in the three visual conditions (eyes open: EO; eyes closed: EC, and with optokinetic stimulation) on the stable platform, before and after rehabilitation with the rotatory chair protocol, in the three groups of UVH patients: the early group (open boxplots), the late 1 group (grey boxplots), and the late 2 group (black boxplots). Each boxplot shows the mean with the 1st and 3rd quartiles, and whiskers indicate the minimum and maximum PII values. The blue heavy horizontal line and the light blue area correspond to the normative values (mean ± SD) recorded in the healthy population under the same experimental conditions. Compared to these normative data, the three groups of patients exhibited significantly higher PII scores (*: p<0.001), but they did not differed significantly from each other before rehabilitation, and they regained PII scores close to the controls after rehabilitation.
The other postural parameters evaluating the energy cost to control posture (SPD), the amplitude of the CoP displacements for which the patient shifts to close-loop control mechanisms (CP amplitude), and the mean frequency of posture stability without corrections as evaluated by the Hausdorff frequency (HF) showed similar changes. They were altered in the three groups of patients tested before rehabilitation, differing significantly from the healthy controls (Table II). The early and late 1 groups of UVH patients spend more energy to stand erect (SPD: p<0001), shift to close-loop control mechanisms for higher CoP displacements (CP amplitude: p<0001), and have more reduced Hausdorff frequency (HF: p<0.001). These changes were enhanced in the EC and Optokinetic conditions compared to the EO condition. The late 2 group showed a rather different pattern compared to the two other ones, with much more increased SPD, CP amplitude and HF. This pattern is typical of subjects with fear of fall who display a stiffness strategy, standing rigid on the platform like a stick.
Table II: Comparison of the postural parameters recorded in the control population and the three groups of unilateral vestibular hypofunction (UVH) patients examined before rehabilitation in the static posturography testThe mean spectral power density (SPD, in decimal Log, ± SD) derived from the wavelet transform of the stabilogram, the amplitude of the critical point (CP amp, in mm2, ± SD) evaluated with the stabilogram diffusion analysis, and the frequency of the Hausdorff points (HF, in Hz, ± SD) calculated with the fractal analysis are provided for each visual condition of the static posturography test (stable support) with: eye open (EO), eyes closed (EC), and optokinetic stimulation (Opto). The normative values recorded in the control population made of 225 sex- and age-matched healthy subjects are given together with those of the early, late 1 and late 2 groups tested before rehabilitation.
| STABLE SUPPORT EYES OPEN | STABLE SUPPORT EYES CLOSED | STABLE SUPPORT OPTOKINETIC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SPD | CP amp | HF | SPD | CP amp | HF | SPD | CP amp | HF | |
| CONTROLS | 72.8 ± 6.5 | 16.3 ± 12.5 | 2.5 ± 1.9 | 76.4 ± 5.9 | 23.3 ± 18.9 | 2.2 ± 1.4 | 72.4 ± 7.4 | 40.7 ± 41.3 | 1.5 ± 1.0 |
| EARLY GROUP BEFORE REHAB | 77.0 ± 7.2 | 49.2 ± 42.4 | 1.5 ± 1.4 | 82.5 ± 6.5 | 158.0 ± 123.4 | 1.6 ± 1.5 | 87.6 ± 14.8 | 315.2 ± 201.7 | 1.1 ± 0.9 |
| LATE 1 GROUP BEFORE REHAB | 76.2 ± 6.0 | 44.1 ± 49.7 | 1.8 ± 1.7 | 78.7 ± 4.6 | 77.5 ± 43.1 | 0.9 ± 0.7 | 97.1 ± 15.4 | 397.6 ± 304.0 | 0.7 ± 0.6 |
| LATE 2 GROUP BEFORE REHAB | 80.5 ± 8.3 | 120.5 ± 125.7 | 3.7 ± 1.9 | 87.1 ± 13.2 | 141.3 ± 101.1 | 2.5 ± 1.8 | 93.7 ± 17.4 | 135.1 ± 131.0 | 1.0 ± 0.6 |
The ANOVAs performed on the posturography parameters after rehabilitation pointed to significant improvements for three of them: the PII [F(1,222) = 100.4;p < 0.0001], the SPD [F(1,222) = 70.3;p < 0.0001], and the CP amplitude [F(1,222) = 37.7; p < 0.0001]. The HF parameter was not significantly modified after rehabilitation [F(1,222) = 1.27; p=0.26]. Whereas the PII regained near normal values in the three groups of UVH patients, whatever the visual condition (cf Fig. 4), the SPD, the CP amplitude and the HF remained altered with EC and with optokinetic stimulation. In this latter condition, for instance, the mean SPD scores were still much higher in the early group (84.7 ± 10.0; p<0.001), the late 1 group (87.4 ± 9.6; p<0.001), and the late 2 group (89.4 ± 12.4; p<0.001) compared to the controls. The mean CP amplitude remained higher also (230.4 ± 202.0, 213.3 ± 242.1, and 266.9 ± 242.5 for the early, late 1 and late 2 groups, respectively: p<0.001), and the HF lower (0.97 ± 0.8, 1.1 ± 0.9, and 1.2 ± 1.0 for the early, late 1 and late 2 groups, respectively; non-significant).
Interesting is the comparison between the early group (having finished its four weeks rehabilitation 35 days after onset of vertigo attack, on average), and the late 2 group (beginning rehabilitation at a similar time period, 44 days on average: see Fig. 1). This comparison makes it possible to differentiate the effects of the natural process of spontaneous vestibular compensation from those of the rehabilitation. Table III illustrates the mean values (± SD) for all the postural parameters in these two groups. The late 2 group without rehabilitation showed significantly higher PII score (worst posture control), increased CP amplitude (closed-loop mechanisms for greater body sway amplitude), and higher SPD value (more energy spent) associated to an abnormally enhanced Hausdorff frequency compared to the rehabilitated early group, in both the EO and EC conditions. These data strongly suggest that the improvement of static posture control in the early group is largely due to the rehabilitation per se.
Table III: Table comparing the postural parameters recorded in the static posturography test between the early group after rehabilitation and the late 2 group before rehabilitationThe data show the mean Postural Instability Index (PII ± SD), Spectral Power Density (SPD ± SD, in decimal Log), Critical Point amplitude (CP amp ± SD, in mm2), and Hausdorff Frequency (HF ± SD, in Hz) in the early group tested after rehabilitation and the late 2 group before rehabilitation, that is, at roughly similar time periods after onset of vestibular disease (~35 days and ~44 days for the early and late 2 group, respectively). Significant differences are indicated with the probability level.
| STABLE SUPPORT EYES OPEN | STABLE SUPPORT EYES CLOSED | STABLE SUPPORT OPTOKINETIC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WAVELET | DIFFUSION | FRACTAL | WAVELET | DIFFUSION | FRACTAL | WAVELET | DIFFUSION | FRACTAL | ||||
| PII | SPD | CP | F | PII | SPD | CP | F | PII | SPD | CP | F | |
| EARLY GROUP AFTER REHAB (~ 35 days) | 1.81 ± 0.6 | 74.3 ± 6.3 | 34.3 ± 29.2 | 1.25 ± 0.9 | 2.33 ± 0.67 | 79.6 ± 5.7 | 81.2 ± 55.3 | 1.10 ± 0.95 | 3.37 ± 1.32 | 84.7 ± 10.0 | 230.4 ± 201.2 | 0.97 ± 0.8 |
| LATE 2 GROUP BEFORE REHAB (~ 44 days) | 3.03 ± 0.85 | 80.5 ± 8.3 | 120.5 ± 125.7 | 3.69 ± 1.9 | 3.31 ± 0.77 | 87.1 ± 13.2 | 141.3 ± 101.1 | 2.53 ± 1.8 | 4.64 ± 2.19 | 93.7 ± 17.4 | 135.1 ± 131.0 | 1.0 ± 0.6 |
| P | 0.0003 | 0.03 | 0.01 | 0.001 | 0.003 | 0.03 | 0.05 | 0.01 | 0.03 | 0.08 | 0.61 | 0.36 |
Dynamic Posturography
The dynamic posturography test consisted of the same visual conditions (EO, EC, Opto) with the patients standing on an unstable support, the platform being free to move in the 3D space. In the most challenging postural tasks without vision or with a moving visual environment, and contrary to what observed in the EO condition, many UVH patients were not able to keep their balance. Figure 5 illustrates the percentage of fallers within each group before and after rehabilitation. The mean percentages did not differ significantly between the groups before rehabilitation, in both the EC (31.8%, 30.8% and 37.5% for the early, late 1 and late 2 groups respectively) and optokinetic condition (68.2%, 84.6% and 75.1% for the same groups, respectively). After rehabilitation, the number of fallers was significantly reduced in the early and late 1 groups only, in both the EC (9% and 15.4%: chi2 = 17.6 and 8.15, respectively; p<0.001 and p<0.02, respectively) and optokinetic (22.7% and 23.1%: chi2 = 9.16 and 9.88, respectively; p<0.02 and p<0.01, respectively). No significant changes were observed after rehabilitation for the late 2 group, whatever the visual condition.
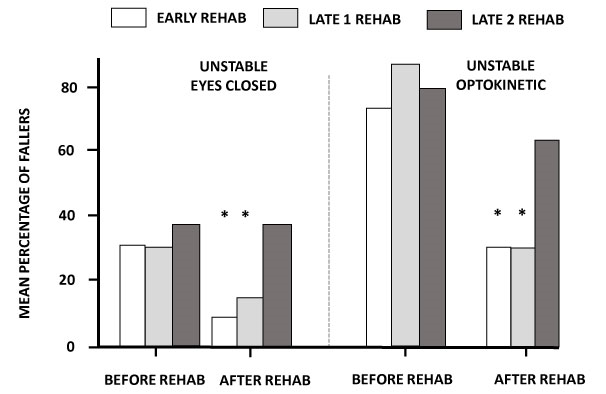
Figure 5: Mean percentage of fallers in the unilateral vestibular hypofunction (UVH) patients before and after rehabilitation in the most challenging dynamic posturography tasks
Illustration of the mean percentage of patients not able to keep balance on the unstable platform with the eyes closed (left histograms) and with optokinetic stimulation (right histograms) before and after rehabilitation in the early group (open histograms), the late 1 group (grey histograms), and the late 2 group (black histograms). Similar percentages were observed in the three groups before rehabilitation, and significantly reduced percentages were found after rehabilitation in the early and late 1 groups. *: p<0.001 and p<0.02, respectively, in the EC condition; and p<0.02 and p<0.01, respectively, in the optokinetic condition.
After rehabilitation, a significant improvement of the postural performance was found in the EO condition in both the early (p<0.02) and late 1 (p<0.05) groups. Patients of the late 2 group showed no significant differences compared to their pre-rehabilitation performance (p=0.38). Many patients were still falling in the optokinetic condition, and particularly in the late 2 group, the statistical analysis was not performed in this condition due to the too low sampling.
Figure 6 illustrates the postural performance of the three groups of patients in the EC condition. It is assumed that this dynamic posturography condition favors the vestibular input since vision is excluded and somatosensory information is not really reliable on an unstable surface. The early group was the only one for which all the parameters investigated by the wavelet analysis (PII, SPD), the diffusion analysis (CP amplitude), and the fractal analysis (HF) were significantly improved after rehabilitation, and the late 2 group was the only one for which none of these parameters was significantly modified. The late 1 group showed an intermediate pattern with significant modifications for some parameters only. For instance, the mean global postural score (PII: 4.31 ± 2.41; Fig. 6A), energy cost (SPD: 96.6 ±10.4; Fig.6B), body sway amplitude without corrections (CP: 349.9 ±197.2; Fig.6C), and frequency of body stabilization (HF: 0.82 ± 0.7; Fig.6D) showed significant improvement in the early group compared to the late 2 group for which the PII (7.72 ± 4.07; p<0.01), the SPS (103.2 ± 15.4; p<0.02), and the CP amplitude (937.6 ± 510.7; p<0.01) were significantly higher, and the HF lower (0.51 ± 0.3; p<0.01). The early group pattern indicates that patients sway less, spend less energy, correct body sway by feedback loop mechanisms for smaller CoP displacements, and have more frequent periods of posture stabilization.
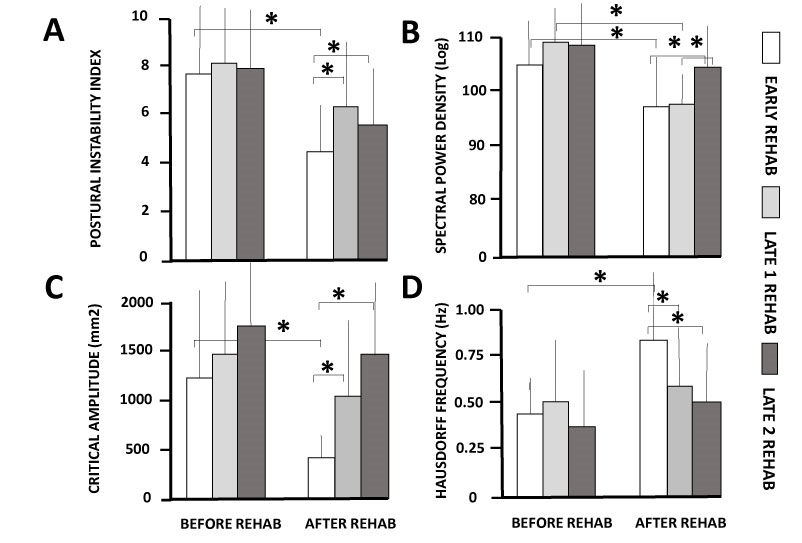
Figure 6A-D: Changes in the postural parameters recorded after rehabilitation in the unilateral vestibular hypofunction (UVH) patients tested in the dynamic posturography tasks
The figure plots the mean modifications of the Postural Instability Index (A), Spectral Power Density (B), Critical Point amplitude (C) and Hausdorff Frequency (D) in the early (open histograms), late 1 (grey histograms), and late 2 (black histograms) groups of patients before (left histograms) and after (right histograms) rehabilitation. Note the significant improvements of all postural parameters observed in the early group only. *: p<0.01.
DISCUSSION
Taken together, the study showed that the postural performance of the UVH patients 1) was impaired before rehabilitation compared to sex- and age-matched healthy controls, 2) was improved after rehabilitation with the rotatory chair protocol, 3) was not dependent of the time delay between onset of pathology and beginning of rehabilitation for the static postural tasks, 4) was better in the early group compared to the late 1 and late 2 groups for the dynamic postural tasks, and 5) patient’s perception of dizziness handicap was reduced in the three groups of patients, with significantly greater reductions when rehab was performed in the early postlesional stages.
Postural performance of the unilateral vestibular hypofunction patients
Everyone agrees that balance control is impaired in patients with unilateral vestibular loss19,47. It is however less trivial to investigate how posture control is altered and recovered in such patients by analyzing the static and dynamic balance function with more functional describers than the simple ones (length and area of CoP displacements) generally used in previous investigations40,43. The postural parameters provided by non-linear analyses of CoP displacements like the wavelet transform and the fractional Brownian-motion analysis give access not only to the global quantification of posture stabilization (PII). They also question the feedback mechanisms controlling body sway (CP amplitude), the energy necessary to maintain quiet standing (SPD) and how often the body is stable in a given time period (HF). All these parameters were significantly altered in our UVH patients examined in static posturography conditions before rehabilitation compared to normative data collected in a population of sex-and age-matched healthy subjects. The PII, the SPD and the CP amplitude were significantly increased and the HF significantly decreased. What these functional postural parameters tell us is that patients have altered quiet standing with or without vision, spend more energy to stabilize body position in space, do postural corrections for greater body sway, and are less frequently stable. The reason why they may fall even in non-challenging postural tasks. The only exception was the late 2 group that showed in the three visual conditions a higher Hausdorff frequency associated to a higher spectral power density, a typical pattern observed in both healthy anxious patients and poorly compensated unilateral vestibular loss patients48. In this latter study, the Short Anxiety Screening Test showed that compensated Menière’s patients after unilateral vestibular neurectomy displayed more anxiety than healthy subjects. Modifying the environmental context by eye closure on an unstable support, and therefore the central representation of the task (higher risk and fear of fall), and / or modifying the posture multisensory integration process by vestibular imbalance, impact both the feedback and feedforward mechanisms controlling posture. Patients can become more tense and more rigid, exhibit a stiffness strategy to reduce their CoP displacements and avoid falls. The absence of rehabilitation can lead to such maladaptive strategy as previously reported by Horak et al.49 (the strap down strategy) and illustrated by the late 2 group.
Rehabilitation of posture and balance with the rotatory chair protocol
The benefit of vestibular rehabilitation therapy (VR) on the postural control of patients with unilateral peripheral dysfunction has been reported many times before25,34,50, and there is a general agreement today to consider that VR helps to resolve symptoms like dizziness and balance impairment23-24,51. This study is the first to report postural improvement in UVH patients rehabilitated with the rotatory chair paradigm. Although used by many French physiotherapists since a long time35,38, this protocol had never been evaluated before.
Postural improvement has been shown in the three groups of patients tested in both the static and dynamic postural tasks, and the first question is to know whether it results from a natural, spontaneous process, or to the impact of VR. There is a well-documented literature reporting vestibular syndrome amelioration over weeks and months through the process of vestibular compensation18,20,21,26,52. The reason why this process may be considered as the neuro-otologist’s best friend53. Overcoming the vestibular disorders has however, a long time constant of several weeks for the static posture recovery and more for the dynamic balance function that remains generally poorly compensated. Comparing the late 2 group before VR and the early group after VR, at roughly similar time-intervals after onset of the pathology (more than 1 month on average) clearly showed that the non-rehabilitated late 2 group had significantly worst postural performance in both the static and dynamic postural tasks. In the easier postural task for example (EO, stable support), the global PII was much higher in the late 2 versus the early group (3.03 ± 0.85 vs 1.81 ±0.6), the energy spent to control posture was significantly much bigger (80.5 ± 8.3 vs 74.3 ± 6.3), and the postural corrections by close-loop control mechanisms occurred for much greater amplitude of body sway (120.5 ±125.7 vs 34.3 ± 29.5, that is, CoP displacements of 10.98 mm vs 5.85 mm). Similar findings were found in the EC and Optokinetic visual conditions (Cf Table III). Moreover, in the dynamic postural tasks, the percentage of fallers was also significantly different in the late 2 group before VR compared to the early group after VR in both the EC (37.5% vs 9%) and Optokinetic (63.8% vs 22.7%) visual conditions. In addition, all the parameters provided by the wavelet transform of the CoP displacements, the diffusion and the Brownian analyses of the stabilogram were significantly improved in the early group after VR compared to the late 2 group before VR (see Fig. 6). Taken together, the data strongly suggest that the spontaneous vestibular compensation process developing in the late 2 group in the period without VR was not enough, if any, to overcome the static and dynamic vestibular deficits. The VR with the rotatory chair protocol was therefore the main source of posture improvement in our UVH patients, interacting very likely with the spontaneous vestibular compensation mechanisms to accelerate the functional recovery26.
Among the sensory substitution mechanisms involved in the natural vestibular compensation process, the visual cues are known since a long time be a strong extra-vestibular input substituting to the lack of vestibular signals48,54-57. The three groups of rehabilitated UVH patients showed worst postural performances when vision was excluded or disturbed under both quiet standing and more challenging conditions, confirming this general statement. More reliance on vision was particularly evidenced by a higher energy cost to control balance, a higher amplitude of body sway without postural corrections, and a lower Hausdorff frequency attesting of stable body positions for greater time-intervals. The poorest postural performance under optokinetic stimulation could reflect a dependence on visual motion cues, frequently observed in vestibular loss patients57.
Is earlier better for the rehabilitation of posture and balance?
We have shown recently that UVH patients rehabilitated with unidirectional rotation paradigms based on either active head35 or passive whole body38 rotations towards the hypofunction side recovered a near normal vestibulo-ocular reflex gain on the disease side when, and only when rehabilitation was performed in the early stage of the pathology. These data corroborated the sensitive period we already demonstrated in animal models28,29. One pressing question from a clinical point of view was therefore to know if such an opportunity time window is present for optimal recovery of posture and balance. The answer seems negative for the recovery of quiet standing while it seems positive for balance recovery in more challenging conditions.
Posture control results from the central integration of multisensory inputs and the internal representation of body orientation in space. This multisensory feedback regulates posture control and continuously updates the internal model of body’s position which in turn forwards motor commands adapted to the environmental context and constraints. It means that posture and balance functions are more complex than the simple VOR, and thus have much more potential vicarious processes to supply the lack of vestibular input. The egocentric reference frame (cutaneous receptors of the solar plant, proprioception from the leg muscles,…) as well as the allocentric reference frame (static vision and visual motion cues), are sensory substitution mechanisms playing a crucial role in the recovery of quiet standing7,13,15,16,21,58. This could explain why posture control in non-challenging conditions can be improved whatever the time delay between onset of the pathology and beginning of rehabilitation. Even though the late 2 group has begun the rehabilitation more than one month after the attack of vertigo, on average, posture improvement with the rotatory chair protocol (and with other rehabilitation methods) remains possible. The only advantage of an early rehabilitation is to accelerate the natural compensation, and to fight maladaptive strategies like the whole body rigidification.
By contrast, balance recovery in challenging conditions with distorted proprioception (unstable support surface) and elimination (EC) or disturbance (optokinetic) of the visual feedback, is much better when rehabilitation is done early. The percentage of fallers is significantly lower in the early and late 1 groups compared to the late 2 group in both the EC and optokinetic conditions. On the other hand, the early group is the only one to show significant improvements for all the postural parameters, while none of them were significantly modified in the late 2 group. The data indicate that dynamic balance control is better restored when VR is performed at an early stage after onset of the pathology, suggesting again a critical period for optimal recovery. This opportunity time window could be explained by synaptic reorganizations at the peripheral59 or central35,38 vestibular levels. Targeting the early period of vestibular lesion-induced neural plasticity with vestibular rehabilitation would lead to the best functional recovery. The reinforcement of vestibulo-spinal pathways for balance control by reweighting of otolith afferents could be the equivalent of the reinforcement of vestibulo-ocular pathways for gaze stabilization by semicircular canal afferents. Interestingly, it has been reported that patients with unilateral vestibular loss who rely on their remaining intact vestibular function showed better postural performance on unstable surface than those who did not22.
Another non-exclusive hypothesis is based on the interactions between stress and vestibular compensation and their causal effect on the patient’s physical activity. A high level of vestibular deafferentation-induced stress response influences the subsequent development of vestibular compensation60, and inadequate vestibular compensation may be responsible for persistent dysfunctions as chronic dizziness57. Many kinds of physical therapy favor the vestibular compensation process25 and reduce the emotional and psychological factors mostly responsible for the patient’s fear of fall. Among the predictors of clinical recovery from vestibular neuritis are the increased visual dependence, anxiety/depression, and fear of bodily sensations57,61. In addition, patients without rehabilitation have longer time of sedentary behavior, shorter time of physical activity62, use maladaptive strategies as head motion avoidance, the reason why the late 2 group has postural scores as low as the early group before rehabilitation and exhibited less recovery after rehabilitation. The subjective evaluation of the quality of life with the DHI questionnaire corroborates this statement.
Taken together, the results of the present study and those of our previous investigations in patients with the same vestibular pathology35,38 strongly suggest that the best functional recovery is always observed when vestibular rehabilitation is started early after the acute attack of vertigo. It means that the critical point to start vestibular rehabilitation is “as soon as possible, as early as the patients can do gaze stabilization exercises or can be submitted to the rotatory chair protocol without exhibiting major discomfort”. Whatever the precise date to begin VR, depending on when the right diagnosis has been established, it is clear that the plasticity mechanisms restoring the functions (full VOR recovery instead of compensatory saccades, for instance) must be used at a very early stage (first two weeks). Regarding posture control, a multisensory determined process, the first four weeks constitute an ideal time window for rehabilitation. After this time, VR will be less effective and/or will require much more training and expensive health costs.
DHI Score
We confirm here previous studies showing that VR improves the DHI score of patients with vestibular hypofunction. Before rehabilitation, all groups were in the same range of moderate handicap (DHI score around 60). After rehabilitation with the rotatory chair protocol, all the patients in the early and late 1 groups showed greater than 18-point difference between pre- and post-rehabilitation scores, a point difference considered as a measure of change with the DHI questionnaire46. The percentage reduction of the DHI score in these groups was roughly similar to that found in our previous study35, but higher (67% and 66% for the early and late 1 groups, respectively) compared to other reports indicating around 35% improvement only63,64. The lower DHI score decrease in these studies in chronic patients results from the longer time period after onset of vestibular pathology, roughly similar to that of the late 2 group for which improvement was 30% only. It reflects very likely the poor recovery of dynamic balance when late rehabilitation is provided. It is another argument supporting the concept that rehabilitation must be done early after the acute attack to get not only the best and faster balance improvement, but also to reduce the patient’s perception of dizziness handicap. Finally, and to plagiarize the Kennard’s principle based on a negative linear relation between age at brain injury and functional outcome (the younger the lesioned organism, the better the outcome65), we could say: the earlier the rehabilitation, the better the dynamic balance recovery; or it’s better to do vestibular rehabilitation early, if you can arrange it.
Limits of the study
It should be necessary to validate our results in larger clinical trials involving more patients with well diagnosed unilateral vestibular hypofunction rehabilitated at different stages of the pathology. This is the first limitation of the study. A second limitation is the short time period of rehabilitation (4 weeks) and the limited number of vestibular rehab sessions (8) provided to each patient. One may wonder if a more intense training would benefit more to the patients to restore their postural performance, and particularly in challenging conditions with suppression of vision, disturbing visual motion cues, and uneven surface. Indeed, these are day life situations frequently experienced by the patients, at the origin of dizziness, instability and fall. Finally, the rotatory chair protocol should be explored more deeply by investigating its contribution on the slow phase eye velocity of the spontaneous nystagmus and the time constant of the vestibular system on the healthy side. This is the future of our running experiments.
Acknowledgments
We thank all the patients for their active participation in the study.
Author contributions
LT diagnosed and selected the patients included in the study; AT did the vestibular rehabilitation of the patients; ML, AT and LT elaborated the experimental protocol; ML wrote the paper; and LT, AT and ML corrected together the manuscript.
Conflict of interest
The authors declare having no conflict of interest.
References
- Massion J. Postural control system. Curr Opin Neurobiol. 1994;4:877-87.
- Mergner T, Rosemeier T. Interaction of vestibular, somatosensory and visual signals for postural control under terrestrial and microgravity conditions: a conceptual model. Brain Res Rev. 1998;118:35.
- Borel L, Lopez C, Péruch P, et al. Vestibular syndrome: a change in internal spatial representation. Neurophysiol Clin Clin Neurophysiol. 2008;38:375-389.
- Lacour M, Borel L. Vestibular control of posture and gait. Arch Ital Biol. 1993;131:81-104.
- Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol (Lond). 1994;478.1:173–186.
- Curthoys IS, McDougall HG, Vidal PP, et al. Sustained and transient vestibular systems: a physiological basis for interpreting vestibular function. Front Neurol. 2017;8:117. Doi: 10.3389/fneur2017.00117.
- Nashner LM, Black FO, Wall C. Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci. 1982;2:536–44.
- Black FO, Wall CII, Nashner LM. Effect of visual and support surface references upon postural control in vestibular deficit subjects. Acta Otolaryngol (Stockh). 1983;95:199-210.
- Dieringer N. Vestibular compensation: neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol. 1995;46:97-129.
- Black FO, Shupert CL, Horak FB, et al. Abnormal postural control associated with peripheral vestibular disorders. In: Pompeiano O, Allum JHJ (eds) Vestibulospinal control of posture and locomotion. Elsevier, Amsterdam. 1988;263-275.
- Black FO, Shupert CL, Peterka RJ, et al. Effects of unilateral loss of vestibular function on the vestibulo-ocular reflex and postural control. Ann Otol Rhinol Laryngol. 1989;98:884-889.
- Borel L, Harlay F, Magnan J, et al. Deficits and recovery of head and trunk orientation and stabilization after unilateral vestibular loss. Brain. 2002;125:880-894.
- Horak FB, Shupert CL. Role of the vestibular system in postural control. In: Herdman SJ (ed) Vestibular rehabilitation. Davis, Philadelphia. 1994;22-46.
- Allum JHJ, Keshner EA, Honegger F, et al. Indicators of the influence a peripheral vestibular deficit has on vestibulo-spinal reflexes controlling postural stability. Acta Otolaryngol (Stockh). 1988;106:252-263.
- Fetter M, Diener HC, Dichgans J. Recovery of postural control after an acute unilateral lesion in humans. J Vest Res. 1991;1:373–383.
- Lacour M, Barthelemy J, Borel L, et al. Sensory strategy in human postural control before and after unilateral vestibular neurectomy. Exp Brain Res. 1997;115:300-310.
- Borel L, Harlay F, Lopez C, et al. Walking performance of vestibular defective patients before and after unilateral vestibular neurotomy. Beh Brain Res. 2004;150:191-200.
- Curthoys IS, Halmagyi GM. Vestibular compensation: clinical changes in vestibular function with time after vestibular loss. In: Buttner U (Ed) Vestibular Dysfunction and its Therapy. Kargel, Basel. 1999.
- Lacour M. Restoration of vestibular function: basic aspects and practical advances for rehabilitation. Curr Med Res Opin. 2006;22:1651-1659. Doi.org/10.1185/03007 9906X 11569 4.
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Rev. 1989;14:155-180.
- Curthoys IS. Vestibular compensation and substitution. Curr Opin Neurol. 2000;13:27-30.
- Horak FB. Postural compensation for vestibular loss. Rest Neurol Neurosci. 2010;28:57-68. Doi: 10.3233/RNN-2010-0515.
- Hillier SL, McDonnell M. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2011;2:CD005397. Doi.org/10.1002/14651 858.CD005 397. pub4.
- Hillier SL, McDonnell M. Is vestibular rehabilitation effective in improving dizziness and function after unilateral peripheral vestibular hypofunction? an abridged version of a Cochrane review. Eur J Phys Rehab Med. 2016;52:541-556. Doi. org/10.1002/14651 858.CD005 397.pub4.
- Whitney SL, Alghwin A, Alghadir A. Physical therapy for persons with vestibular disorders. Cur Op Neurol. 2015;28:162. Doi: 10.1097/wco00162.
- Lacour M, Bernard-Demanze L. Interaction between vestibular compensation mechanisms and vestibular rehabilitation therapy. 10 recommendations for optimal functional recovery. Front Neurol. 2014;5:285-297.
- Hall CD, Herdman SJ, Whitney SL, et al. Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guidelines. J Neurol Phys Ther. 2016;40:124-155. Doi.org/10.1097/NPT.00000 00001 20.
- Lacour M, Roll JP, Appaix M. Modifications and development of spinal reflexes in the alert baboon (Papio Papio) following an unilateral vestibular neurectomy. Brain Res. 1976;113:255-269.
- Xerri C, Lacour M. Role of sensorimotor activity in compensating posturo-kinetic deficits after unilateral vestibular neurectomy in the cat. Acta Otolaryngol. 1980;90:414-424.
- Meldrum D, Jahn K. Gaze stabilisation exercises in vestibular rehabilitation: review of the evidence and recent clinical advances. J Neurol. Sep 2019;266(Suppl 1):11-18. doi: 10.1007/s00415-019-09459-x. Epub 2019 Aug 5.
- Enticott JC, O’leary SJ, Briggs RJS. Effects of vestibulo-ocular reflex exercises on vestibular compensation after vestibular schwannoma surgery. Otol Neurotol. 2005;26:265-269.
- Teggi R, Caldirola D, Fabiano B, et al. Rehabilitation after acute vestibular disorders. J Laryngol Otol. 2009;123:397-402.
- Topuz O, Topuz B, Ardic FN, et al. Efficacy of vestibular rehabilitation on chronic unilateral vestibular hypofunction. Clin Rehabil. 2004;18:76–83. Doi.org/10.1191/02692 15504 cr704 oa.
- Herdman SJ, Hall CD, Delaune W. Variables associated with outcome in patients with unilateral vestibular hypofunction. Neurorehabilit Neural Repair. 2012;26:151-162.
- Lacour M, Tardivet L, Thiry A. Rehabilitation of dynamic visual acuity in patients with unilateral vestibular hypofunction: earlier is better. Eur Arch Otorhinolaryngol. 2020a;277:103-113. Doi: 10.1007/s00405-019-05690-4.
- Ushio M, Minor LB, Della Santina CC, et al. Unidirectional rotations produce asymmetric changes in horizontal VOR gain before and after unilateral labyrinthectomy in macaques. Exp Brain Res. 2011;210:651-660. Doi.org/10.1007/ s0022 1-011-2622-2.
- Sadeghi NG, Azad BS, Rassian N, et al. Rebalancing the vestibular system by unidirectional rotations in patients with chronic vestibular dysfunction. Front Neurol. 2018. Doi.org/10.3389/fneur .2018.01196.
- Lacour M, Tardivet L, Thiry A. A critical period for rehabilitation of unilateral vestibular hypofunction patients with the unidirectional rotation paradigm. J Rehab Therapy. (2020b).2(1):16-22.
- Strupp M, Magnusson M. Acute unilateral vestibulopathy. Neurol Clin. 2015;33:669–685. Doi.org/10.1016/J.ncl.2015.04.012.
- Lacour M, Bernard-Demanze L, Dumitrescu M. Posture control, aging, and attention resources: models and posture analysis models. Clin Neurophysiol. 2008;38:411-421.
- Bernard-Demanze L, Leonard J, Dumitrescu M, et al. Static and dynamic posture control in postlingual cochlear implanted patients: effects of dual-tasking, visual and auditory inputs suppression. Front Int Neurosci. 2014;7:111. Doi.10.3389/fnint.2013.00111.
- Bernard-Demanze L, Lacour M. The fall in older adults: physical and cognitive problems. Current Aging Sciences. 2017;10(3):185-200.
- Lacour M, Yavo Dusso N, Thiry A, et al. How eye movements stabilize posture in patients with bilateral vestibular hypofunction. Frontiers in Neurology. 2018;9:744. Doi.org/103389/fneur.2018.00744.
- Collins JJ, De Luca CJ. Open-loop and close-loop control of posture: a random-walk analysis of center-of-pressure trajectories. Exp Brain Res. 1993;95:308-18. Doi: 10.1007/BF00229788.
- Einstein A. Uber die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flussigkeiten suspendierten Teilchen. Ann Phys. 1905;322:549-560.
- Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424-427. Doi.org/10.1001/archo tol.1990.01870 040046011.
- Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vest Res. 1995;5:67-107.
- Young L, Bernard-Demanze L, Dumitrescu M, et al. Postural performance of vestibular loss patients under increased postural threat. J Vest Res. 2012;22:129-138. Doi.10.3233/VES-2012-0449.
- Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82:167–177.
- Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: Review of indications, mechanisms and key exercises. J Clin Neurol. 2011;7:184-196.
- McDonnell MN, Hillier SL. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2015;1:CD005397.
- Lacour M, Tighilet B. Plastic events in the vestibular nuclei during vestibular compensation: the brain orchestration of a deafferentation code. Rest Neurol Neurosci. 2010;28:19-35. Doi.org/10.3233/RNN-2010-0509.
- Lacour M, Helmchen C, Vidal PP. Vestibular compensation: the neuro-otologist’s best friend. J Neurol. 2015. Doi: 10.1007/s00415-015-7903-4.
- Dichgans J, Mauritz KH, Allum JH, et al. Postural sway in normal and atactic patients: analysis of the stabilising and destabilizing effects of vision. Agressologie. 1976;17:15–24.
- Paulus WM, Straube A, Brandt T. Visual stabilization of posture: physiological stimulus characteristics and clinical aspects. Brain. 1984;107:1143–63.
- Diener HC, Dichgans J, Guschlbauer B, et al. Role of visual and static vestibular influences on dynamic posture control. Hum Neurobiol. 1986;5:105-13.
- Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol. Neurosurg. Psychiatry. 1995;59:472-476. Doi: 10.1136/jnnp.59.5.472.
- Black FO, Nashner LM. Vestibulo-spinal control differs in patients with reduced versus distorded vestibular function. Acta Otolaryngol (Stockh). 1984;[Suppl] 406:110-114.
- Gaboyard-Niay S, Travo C, Saleur A, et al. Correlation between afferent rearrangements and behavioral deficits after local excitotoxic insult in the mammalian vestibule: a rat model of vertigo symptoms. Disease Models and Mechanisms. 2016;ç:1181-1192. Doi:10.1242/dmm.024521.
- Saman Y, Bamlou DE, Gleeson M, et al. Interactions between stress and vestibular compensation: a review. Front Neurol. 2012;3:116. Doi: 10.3389/fneur.2012.00116.
- Cousins S, Kaski D, Cutfield N, et al. Predictors of clinical recovery from vestibular neuritis: a prospective study. Ann Clin Translat Neurol. 2017;4:340-346. Doi: 10.1002/acn3.386.
- Morimoto H, Asai Y, Johnson EG, et al. Objective measures of physical activity in patients with chronic unilateral vestibular hypofunction, and its relationship to handicap, anxiety and postural stability. Auris Nasus Larynx. 2018. Doi: org/10.1016/j.
- Szturm T, Ireland DJ, Lessing-Turner M. Comparison of different exercise programs in the rehabilitation of patients with chronic peripheral vestibular dysfunction. J Vest Res. 1994;4:461–479.
- Wai Yip C, Strupp M. The dizziness handicap inventory does not correlate with vestibular function tests: a prospective study. J Neurol. 2018;265:1210-1218. Doi.org/10.1007/s00415-018-8834.7.
- Kennard M. Age and other factors in motor recovery from precentral lesions in monkeys. Am J Physiol. 1936;115:138-146.
