Exercise activates AMPK signaling: Impact on glucose uptake in the skeletal muscle in aging
Renan Fudoli Lins Vieira1, Rafael Lima Junqueira1, Rafael C. Gaspar1, Vitor R. Munoz1, Jose R. Pauli1,2*
1Department of Health Sciences, Laboratory of Molecular Biology of Exercise, School of Applied Sciences, University of Campinas, Sao Paulo, Brazil
2CEPECE-Center of Research in Sport Sciences, School of Applied Sciences, University of Campinas (UNICAMP), Limeira, Sao Paulo, Brazil
Abstract
Aging is characterized by a decline in metabolism and functional capacities. On the other hand, physical exercise is a well-known strategy that provides metabolic and functional benefits. Since elderly subjects have insulin resistance and issues with carbohydrate metabolism, physical exercise can contribute to glucose homeostasis through its participation in skeletal muscle glucose uptake via insulin-dependent/insulin-independent mechanisms. Therefore, it is important to understand the effects of physical exercise on 5' AMP-activated protein kinase (AMPK) signaling in skeletal muscle and on positive metabolic adaptations and the prevention of age-related diseases. In this mini-review, we will discuss the participation of AMPK in controlling skeletal muscle glucose uptake in response to physical exercise and aging.
Introduction
The aging process has been widely studied from different perspectives in the scientific community. Aging is associated with the appearance of innumerable diseases and co-morbidities, especially in those with a lifestyle involving poor quality food and physical inactivity. One of the common causes of some of these age-related comorbidities is impaired insulin sensitivity and the downregulation of skeletal muscle glucose uptake, which contributes to impairments in whole-body glucose homeostasis1. Since skeletal muscle is responsible for up to 70–90% of blood glucose uptake in the post-prandial state, the effects of aging and insulin resistance in this tissue may affect its functionality and metabolic capacity, leading to negative effects on glucose homeostasis2,3.
Physical exercise is very well described for its role in the upregulation of skeletal muscle insulin sensitivity and glucose uptake, through mechanisms that are dependent and independent of insulin4. Regarding the insulin-independent glucose uptake mechanisms, AMPK has shown important participation in the skeletal muscle glucose uptake independently of insulin, being widely studied because due to its varied benefic effects on metabolism. Previous studies have shown the role of AMPK in the stimulation of skeletal muscle glucose uptake in the absence of insulin, and also in promoting fatty acid oxidation and mitochondrial biogenesis5-7. AMPK activity is also associated with autophagy activation and inhibition of mechanistic target of rapamycin (mTOR), a protein that when upregulated is involved with age-related comorbidities8. The benefic effects of exercise-induced AMPK activation in the skeletal muscle in the condition of advanced age will be reviewed in this mini-review9.
AMPK actions in metabolism
AMPK is regulated by the levels of adenosine monophosphate (AMP) and adenosine triphosphate (ATP) inside the cell, acting as an energy sensor. The heterotrimeric structure of this protein is composed of the α catalytic subunit, and the regulatory β and γ subunits. Although some studies suggest that the α2 catalytic subunit is essential to skeletal muscle glucose uptake, some other studies suggest that the regulatory subunits (β2 and γ3) are necessary to stimulate glucose uptake in response to AMPK activators10. Since AMPK acts as a crucial cellular energy sensor, the activation of catabolic mechanisms (glucose uptake/glycolysis, lipolysis, autophagy, mitophagy) and inactivation of anabolic mechanisms (lipogenesis, glycogenolysis, gluconeogenesis, protein synthesis) are involved11,12.
In skeletal muscle, the glucose uptake in response to AMPK activation occurs independently of insulin to stimulate the glucose transporter 4 (GLUT4) vesicle trafficking to the cellular membrane. The mechanism involved in this process is tre-2/USP6, BUB2, cdc16 domain family member 1 (TBC1D1) and Akt substrate of 160 kDa (AS160, also known as TBC1D4) phosphorylation by AMPK, which activate Rab GTPases and stimulate the translocation of GLUT4 vesicles to the membrane13,14. However, a recent study showed the role of AMPK in the regulation of insulin-stimulated glucose uptake15. The authors deleted the protein kinase B (Akt2) in the skeletal muscle of mice and observed no changes in the lean mass and skeletal muscle insulin sensitivity. The deletion of Akt1/2 led to decreased lean mass, but normal insulin-stimulated glucose uptake in the skeletal muscle. Moreover, the chronic Akt ablation induced AMPK activity in response to energetic stress, regulating the skeletal muscle insulin sensitivity. This model was confirmed with an experiment where insulin stimulated the in vivo and ex vivo glucose uptake in Akt1/2 knockout mice; however, the additional treatment with compound C (an AMPK inhibitor) blunted the insulin-stimulated glucose uptake15. Thus, the connection between AMPK and the insulin signaling pathway in skeletal muscle should be investigated in future studies to answer some of the remaining questions.
Besides the role of AMPK in GLUT4 vesicle translocation to the cellular membrane, AMPK induces mRNA expression for GLUT4 and hexoquinase 2 (HK2), an essential enzyme that mediates the glycolysis flux16. In addition, AMPK inhibits glycogen synthesis through glycogen synthase (GS) inhibition17. Moreover, treatment with activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) (a pharmacological AMPK activator) increases glycogen synthase kinase (GSK) phosphorylation, facilitating glycogenolysis in the skeletal muscle18.
Fatty acid oxidation is one of the pathways regulated by AMPK activity. When activated, AMPK phosphorylates acetil-CoA carboxylase (ACC), a protein that controls the conversion of acetil-CoA to malonil-CoA19. This conversion is involved in fatty acid synthesis; however, this shifting mediated by ACC phosphorylation favors fatty acid oxidation to provide substrate availability to myocytes20. Therefore, it is essential to highlight the importance of AMPK in this pathway. The effect of AMPK in increasing the oxidative capacity of skeletal muscle appears to occur through transcriptional regulation. The increase in the content of peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1ï¡ï© and other transcription factors are involved in the process of fat oxidation through AMPK in muscle tissue21.
The chronic activation of mammalian target of rapamycin (mTOR) is related to insulin resistance, tissue degeneration, and tumor development22. AMPK plays an important role in mTOR inhibition in response to energy depletion, through its association with liver kinase B1 (LKB1) and the consequent phosphorylation of tuberous sclerosis complex 2 (TSC2), a protein that inhibits mTOR activity22. At the same time, AMPK mediates important steps of autophagy activation through unc-51 like autophagy activating kinase 1 (ULK1) phosphorylation. In a fed state, mTOR complex phosphorylates ULK1 in inhibitory sites, blocking autophagy. On the other hand, under starvation conditions, these inhibitory sites in ULK1 are dephosphorylated by mTOR complex. ULK1 is also a target of AMPK, showing positive regulation of autophagy by ULK1 phosphorylation. Thus, AMPK indirectly activates autophagy by regulating mTOR inhibition, but also by direct phosphorylation of ULK1 in mammals23. In this regard, activation of AMPK in skeletal muscle through exercise can be an efficient strategy for achieving metabolic health and preventing disease.
Role of physical exercise in AMPK activity
The importance of physical exercise throughout life to provide a healthier elderly life is very well described24. One of the most well-known metabolic responses to physical exercise is the upregulation of skeletal muscle glucose uptake through direct mechanisms that improve insulin sensitivity and insulin-independent mechanisms that also contribute to glucose homeostasis25. In addition, the enhanced energy expenditure promoted by physical exercise reduces intracellular ATP levels and increases AMP levels, inducing AMPK phosphorylation/activation and stimulating skeletal muscle glucose uptake26.
As mentioned before, AMPK is composed of trimeric complexes (α, β, γ) that are differently influenced by exercise duration and intensity9. The α1β2γ1 and α2β2γ1 complexes are activated under moderate intensity exercise with 1 hour of duration27. On the other hand, the α2β2γ3 complex is activated by intense exercise with 20 minutes of duration9. The α2 subunit is also phosphorylated during prolonged exercise, but is not sufficient to maintain ACC phosphorylation in the skeletal muscle of humans28. Well-trained individuals showed increased AMPK phosphorylation after physical exercise; however, the stimulus was different from that of the prior training modality29. In this study, Coffey et al. investigated strength- and endurance-trained individuals and showed that AMPK phosphorylation increased after cycling in the strength-trained group, but not in the endurance-trained group. When the endurance-trained individuals were subjected to resistance exercise, AMPK phosphorylation was increased, something that did not happen in the resistance-trained group29. Finally, the AMPK activity in response to physical exercise depends of the AMP levels independently of the exercise duration and intensity; however, increases in AMP levels are probably higher when the physical exercise is performed until exhaustion30.
Besides the acute effects of physical exercise, chronic physical exercise also induces AMPK activity and PGC-1α, contributing to mitochondrial biogenesis, energy homeostasis, thermoregulation, and glucose metabolism31. In addition, AMPK stimulates the peroxisome proliferator-activated receptor α PPARα mRNA expression, leading to molecular modulations that favor fatty acid oxidation in skeletal muscle31. Therefore, it is of great importance for the elderly to engage in a regular exercise program in order to maintain health and prevent disease. In addition, at older ages, there is usually a decrease in insulin secretion, muscle atrophy, and hemodynamic changes, all of which have implications for the glucose uptake process through insulin signaling32. Therefore, AMPK activation and exercise-mediated glucose uptake is of great importance in aging. This suggests that the elderly should exercise more frequently, in order to promote the activation of AMPK and obtain the benefits associated with this signaling pathway and glucose uptake independent of insulin.
The role of physical exercise in promoting AMPK activation also results in autophagy stimulation. A family of proteins called sestrins are responsive to stress, and may be one mechanism that mediates the effects of physical exercise on AMPK phosphorylation, and consequently mTOR inhibition and autophagy stimulation33. The adequate coordination of these mechanisms by AMPK should slow down some aspects of aging such as tissue degeneration and tumor growth in advanced ages34. Another mechanism involved in AMPK activation by physical exercise is the increase in intracellular calcium (Ca2+) content through membrane depolarization, inducing the calcium/calmodulin-dependent protein kinase kinase (CaMKK) phosphorylation, which can phosphorylate and activate AMPK35,36. Additional information regarding the role of Ca2+ in skeletal muscle glucose uptake was reviewed by Jensen and co-workers37. Moreover, endoplasmic reticulum stress in response to skeletal muscle contraction increases reactive oxygen species (ROS), which leads to CaMKK activation30,38,39. The increase in muscle temperature may also play a role in AMPK activation during physical exercise40. In summary, there is different evidence regarding the effects of physical exercise on AMPK activation through different mechanisms.
Effects of physical exercise and AMPK during aging
It has been shown that the activation of AMPK through exercise can promote metabolic health with advancing age. Previous animal studies have shown that physical exercise stimulates AMPK, as well as skeletal muscle glucose uptake. Wang and colleagues (2016) observed that older (30-month old) rats showed increased skeletal muscle AMPK phosphorylation in response to acute swimming exercise combined with caloric restriction when compared to control animals that performed only caloric restriction or at ad libitum41. Wang et al, also observed increased skeletal muscle glucose uptake in the groups that performed caloric restriction and physical exercise combined with caloric restriction compared to control groups41. These findings suggest that physical exercise can potentiate the effects of caloric restriction on AMPK phosphorylation; however, the same pattern of skeletal muscle glucose uptake observed in the sedentary and exercised groups with caloric restriction could be addressed by the single session of exercise, and not a chronic intervention. The study of Coqueiro et al., observed the effects of preventive and therapeutic aerobic exercise on the soleus and gastrocnemius muscles and cardiometabolic outcomes. It was observed that increases in AMPK gene levels in the soleus muscle, but not in gastrocnemius, of the preventive exercise group was associated with improved cardiometabolic health and glucose homeostasis. This study highlight the importance of long-term exercise in blood glucose homeostasis and longevity42. On the other hand, the therapeutic exercise improved glucose tolerance; however, it was not able to modulate AMPK and improve insulin sensitivity in the case of obesity and aging42. Moreover, the researchers found higher AMPK levels in the soleus muscle oxidative fibers than other skeletal muscle tissue42.
In the study of Mortensen et al, it was evaluated the mRNA and protein levels of AMPK in the skeletal muscle of 100 young and 82 older mono- and dizygotic nondiabetic twins43. Firstly, the authors observed similar mRNA and proteins levels of AMPK between the twin samples, indicating minor genetic influence on skeletal muscle AMPK levels. There was a positive correlation AMPK γ3 mRNA and protein expression, and between AMPK γ3 protein and AMPK γ3 activity. The AMPK γ3 protein levels showed a negative correlation with MHC type 1 mRNA levels, and a positive correlation with type 2 MHC type 2a and 2x mRNA levels. In the older twin, there was lower levels of AMPK γ3 mRNA, protein and activity compared to young individuals, but not in the other AMPK subunits (α1 and α2). It was also observed negative correlation between AMPK γ3 and glycogen content43. This study highlights the importance of environmental factors on AMPK activity, such as age, sex and aerobic capacity.
Aging-related sarcopenia is characterized by a loss of muscle mass and its functionality. There is strong evidence that muscle mass in elderly subjects has a negative correlation with mortality44,45. In addition, is important to highlight the association between lower muscle mass and impaired glucose metabolism in humans46. Thus, physical exercise is the most effective way to prevent this muscle loss44. A study using 19-month old mice showed that AMPK activation in response to physical exercise (4 weeks of treadmill exercise) reduced phenotypes of cellular senescence, by decreasing senescence markers (p16 and p21) compared to sedentary mice47. Moreover, an interesting finding of this study was that physical exercise increased AMPK phosphorylation in the skeletal muscle of young mice; however, the old exercised group showed only a minor increase in AMPK phosphorylation compared to the old sedentary group47. This result may suggest an impaired AMPK response to physical exercise in aged mice.
Although the studies discussed here focus on the effects of physical exercise and AMPK activity in the control of skeletal muscle glucose uptake, there is a correlation with AMPK and positive outcomes in other metabolic tissues such as liver, adipose tissue, endothelium, and hippocampus48-50. In addition, AMPK is also responsive to physical exercise interventions in these tissues48-50.
Therefore, the AMPK pathway is an important mediator of glucose homeostasis through its positive role in skeletal muscle glucose uptake, affecting insulin-dependent/insulin-independent mechanisms (Figure 1). Moreover, AMPK contributes to the benefic effects of physical exercise on whole-body glucose homeostasis. In the aging process, this relationship between exercise and AMPK is extremely positive for metabolic health and the prevention of age-related diseases. Finally, the known effects of physical exercise as a therapeutic strategy to combat obesity, type 2 diabetes, metabolic syndrome, and cardiovascular diseases, may be due to some of the molecular effects of AMPK described here. The current knowledge indicates that moderate and high intensity exercises can activate AMPK in relation to rest and low intensity exercise conditions; however, further studies are needed to investigate which type of exercise is capable of being more efficient in activating AMPK in the elderly. Future studies investigating older population groups and different types and characteristics of exercise will provide more information about AMPK activity in skeletal muscle, and its contribution to whole-body glucose homeostasis.
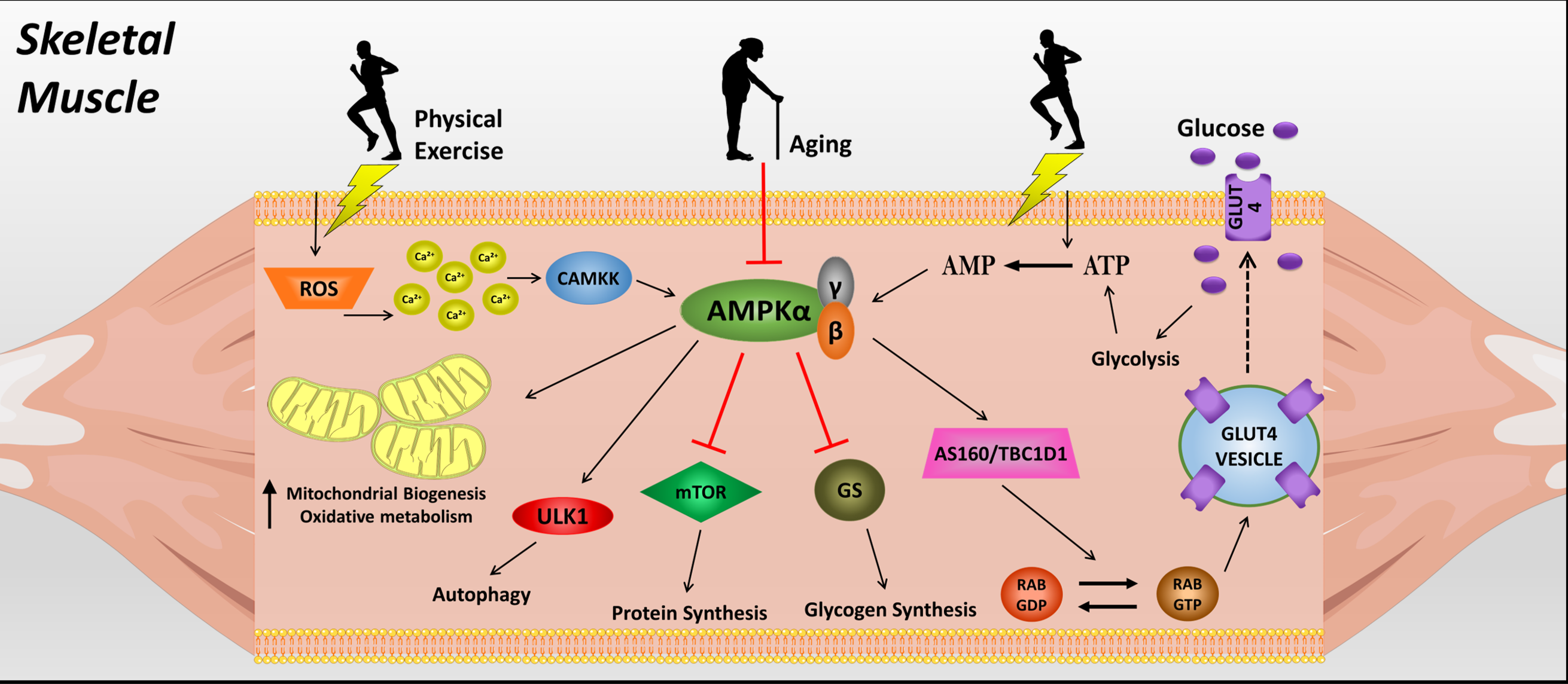
Figure 1: Mechanisms of skeletal muscle glucose uptake mediated by AMPK.
References
- Facchini FS, Hua N, Abbasi F, et al. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574–3578.
- DeFronzo RA, Ferrannini E, Sato Y, et al. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest. 1981. doi:10.1172/JCI110399
- Baron AD, Brechtel G, Wallace P, et al. Rates and tissue sites of non-insulin- and insulin- mediated glucose uptake in humans. Am J Physiol Endocrinol Metab. 1988. doi:10.1152/ajpendo.1988.255.6.e769
- Kjobsted R, Roll JLW, Jorgensen NO, et al. AMPK and TBC1D1 regulate muscle glucose uptake after, but not during, exercise and contraction. Diabetes. 2019;68:1427-1440.
- Stephens TJ, Chen ZP, Canny BJ, et al. Progressive increase in human skeletal muscle AMPKα2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282:688–694.
- Zong, H, Ren JM, Young LH, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 2002;99:15983–15987.
- Pereira RM. Molecular mechanisms of glucose uptake in skeletal muscle at rest and in response to exercise. Motriz Rev Educ Fis. 2017;23:1–8.
- Barzilai N, Huffman DM, Muzumdar RH, et al. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–22.
- Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem J. 2009;418:261–275.
- Sylow L, Kleinert M, Richter EA, et al. Exercise-stimulated glucose uptake - regulation and implications for glycaemic control. Nat Rev Endocrinol. 2016;13:133–148.
- Ross FA, Jensen TE, Hardie DG. Differential regulation by AMP and ADP of AMPK complexes containing different γ subunit isoforms. Biochem J. 2016;473:189–199.
- Garcia D, Shaw RJ. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell. 2017;66:789–800.
- Li J, Zhong L, Wang F, et al. Dissecting the role of AMP-activated protein kinase in human diseases. Acta Pharm Sin. 2017;B7:249–259.
- Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab. 2008;295:29–37.
- Jaiswal N, Gavin MG, Quinn WJ 3rd, et al. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol Metab. 2019;28:1–13.
- Stoppani J, Hildebrandt AL, Sakamoto K, et al. AMP-activated protein kinase activates transcription of the UCP3 and HKII genes in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283.
- Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245.
- Vitzel KF, Bikopoulos G, Hung S, et al. Chronic Treatment with the AMP-Kinase Activator AICAR Increases Glycogen Storage and Fatty Acid Oxidation in Skeletal Muscles but Does Not Reduce Hyperglucagonemia and Hyperglycemia in Insulin Deficient Rats. PLoS One. 2013;8:1–9.
- Matthews VB, Astrom MB, Chan MHS, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418.
- Chang H, Kwon O, Shin MS, et al. Role of Angptl4/Fiaf in exercise-induced skeletal muscle AMPK activation. J Appl Physiol. 2018;125:715–722.
- Thomson DM, Winder WW. AMP-activated protein kinase control of fat metabolism in skeletal muscle. in Acta Physiologica. 2009. doi:10.1111/j.1748-1716.2009.01973.x
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484.
- Alers S, Loffler AS, Wesselborg S, et al. Role of AMPK-mTOR-Ulk1/2 in the Regulation of Autophagy: Cross Talk, Shortcuts, and Feedbacks. Mol Cell Biol. 2012;32:2–11.
- Cartee GD, Hepple RT, Bamman MM, et al. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016;23:1034–1047.
- Miyamoto L. Significance of 5’AMP-activated protein kinase in metabolomic regulation by skeletal muscle contraction. J Phys Fit Sport Med. 2015;4:93–102.
- Radhakrishnan J, Baetiong A, Kaufman H, et al. Improved exercise capacity in cyclophilin-D knockout mice associated with enhanced oxygen utilization efficiency and augmented glucose uptake via AMPK-TBC1D1 signaling nexus. FASEB J. 2019;33:11443–11457.
- Treebak JT. AS160 phosphorylation is associated with activation of α 2β2γ1- but not α 2β2γ3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007;292:715–722.
- Wojtaszewski JFP, Mourtzakis M, Hillig T, et al. Dissociation of AMPK activity and ACCβ phosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun. 2002;298:309–316.
- Coffey VG, Zhong Z, Shield A, et al. Early signaling responses to divergent exercise stimuli in skeletal muscle from wellâtrained humans. FASEB J. 2006;20:190–192.
- Morales-Alamo D, Calbet JAL. AMPK signaling in skeletal muscle during exercise: Role of reactive oxygen and nitrogen species. Free Radic Biol Med. 2016;98:68–77.
- Lee WJ, Kim M, Park HS, et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARα and PGC-1. Biochem Biophys Res Commun. 2006;340:291–295.
- Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748.
- Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013;18:792–801.
- Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–241.
- O’Neill HM. AMPK and exercise: Glucose uptake and insulin sensitivity. Diabetes Metab J. 2013;37:1–21.
- Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245.
- Jensen TE, Angin Y, Sylow L, et al. Is contraction-stimulated glucose transport feedforward regulated by Ca2+? Exp Physiol. 2014. doi:10.1113/expphysiol.2014.081679
- Feng N, Wang B, Cai P, et al. ZEA-induced autophagy in TM4 cells was mediated by the release of Ca2+ activates CaMKKβ-AMPK signaling pathway in the endoplasmic reticulum. Toxicol Lett. 2020;323:1–9.
- Jensen TE, Rose AJ, Jorgensen SB, et al. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007;292:1308–1317.
- Koshinaka K, Kawamoto E, Abe N, et al. Elevation of muscle temperature stimulates muscle glucose uptake in vivo and in vitro. J Physiol Sci. 2013;63:409–18.
- Wang H, Sharma N, Arias EB, et al. Insulin Signaling and Glucose Uptake in the Soleus Muscle of 30-Month-Old Rats After Calorie Restriction With or Without Acute Exercise. Journals Gerontol Ser A Biol Sci Med Sci. 2016;71:323–332.
- Coqueiro R da S, Soares T de J, Pereira R, et al. Therapeutic and preventive effects of exercise on cardiometabolic parameters in aging and obese rats. Clin Nutr ESPEN. 2019;29:203–212.
- Mortensen B, Poulsen P, Wegner L, et al. Genetic and metabolic effects on skeletal muscle AMPK in young and older twins. Am J Physiol Endocrinol Metab. 2009;297:956–964.
- Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–537.
- de Santana FM, Domiciano DS, Goncalves MA, et al. Association of Appendicular Lean Mass, and Subcutaneous and Visceral Adipose Tissue With Mortality in Older Brazilians: The São Paulo Ageing & Health Study. J Bone Miner Res. 2019;34:1264–1274.
- Kalyani RR, Metter EJ, Ramachandran R, et al. Glucose and Insulin Measurements From the Oral Glucose Tolerance Test and Relationship to Muscle Mass. Journals Gerontol Ser A Biol Sci Med Sci. 2012;67ª:74–81.
- Yoon KJ, Zhang D, Kim SJ, et al. Exercise-induced AMPK activation is involved in delay of skeletal muscle senescence. Biochem Biophys Res Commun. 2019;512:604–610.
- Ko JR, Seo DY, Park SH, et al. Aerobic exercise training decreases cereblon and increases AMPK signaling in the skeletal muscle of STZ-induced diabetic rats. Biochem Biophys Res Commun. 2018;501:448–453.
- Lin J, Kuo W, Baskaran R, et al. Swimming exercise stimulates IGF1 / PI3K / Akt and AMPK / SIRT1 / PGC1 α survival signaling to suppress apoptosis and inflammation in aging hippocampus. 2020;12:1–13.
- Chen Z, Yu J, Fu M, et al. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem Pharmacol. 2020;177:113951.
