A critical period for rehabilitation of unilateral vestibular hypofunction patients with the unidirectional rotation paradigm
Michel Lacour1*, Laurent Tardivet2, Alain Thiry3
1Neurosciences Department, Aix-Marseille University/CNRS, Marseille, France
2Otorhinolaryngology Department, CHU Nice, 30 Voie Romaine, 06000 Nice, France
3Physiotherapist, 29 Bd Dubouchage, 06000 Nice, France
Abstract
Unilateral vestibular hypofunction (UVH) patients were submitted to a vestibular rehabilitation (VR) program with two different protocols based on the unidirectional rotation paradigm. One group (N=28) was submitted to active gaze stabilization exercises with the head impulse test (HIT), and a second group (N=31) with the passive whole-body rotation on a rotatory chair. Head or body rotations were always performed to the hypofunction side and a similar number of training sessions were used in each group (2 times a week for four weeks). Patients in each group were subdivided into three subgroups based on the time delay between onset of the disease and beginning of VR (early VR: the first two weeks after onset; late 1 VR: third and fourth weeks after onset; late 2 VR: one month and more after onset). The angular vestibulo-ocular reflex (aVOR) and the directional preponderance (DP) regarding the horizontal canals were the main outcomes. The results pointed to similar findings with the two protocols, characterized by a significant improvement of the aVOR gain on the hypofunction side, responsible for the significant decrease of the DP in the horizontal canals. These powerful changes were observed in the early subgroups only. No significant modifications were found in the late 1 and late 2 subgroups. The data clearly attest to the effectiveness of the unidirectional rotation paradigm when performed in the acute phase of the disease, thus extending to UVH patients the concept of critical period for VR already demonstrated in animal models.
Background
Acute unilateral vestibular hypofunction (UVH) induces in both animals and humans many vestibular symptoms including spinning vertigo, spontaneous nystagmus, posture and gait imbalance, oscillopsia, and associated neuro-vegetative symptoms (nausea and vomiting)1. Most of the static deficits like spontaneous vestibular nystagmus and head tilt fully recover over weeks or months in a process referred to vestibular compensation while the dynamic deficits poorly recover, as illustrated by the angular vestibular ocular reflex2. It has been shown however that brain reorganizations can help the patients to recover their dynamic vestibular functions by means of sensory substitution mechanisms3, new behavioural strategies4,5, and changes in the brain functional connectivity as well6. If there is really a spontaneous compensation of the vestibular deficits7, the time to achieve the full recovery remains however relatively long (up to one year in UVH patients) and the final level of compensation is not optimal in many cases due to inappropriate strategies or avoidance behaviors implemented by the patients themselves8,9. The vestibular rehabilitation therapy (VR) is recognized today as a safe and effective tool to improve balance, dizziness and quality of life in vestibular loss patients10,11. VR accelerates the functional compensation by shortening the time constant of the recovery process, and it optimizes the final level of vestibular compensation12.
Vestibular rehabilitation and the unidirectional rotation paradigm
Unidirectional rotation of the patient’s whole body towards the lesion side was first proposed at the end of the XXth century by Alain Semont, a French physiotherapist, as a clinical tool for rehabilitation of UVH patients. This so-called rotatory chair protocol was used to reduce the response from the intact labyrinth and, therefore, to decrease the vestibular asymmetry seen just after an acute unilateral vestibular loss. This VR protocol is still currently used in France and, according to the physiotherapists, it seems to work. Unfortunately, there are no publications in referred journals to support this method which has been ignored outside. In 2011, Ushio et al.13 provided the first demonstration in chronic unilateral labyrinthectomized macaques that unidirectional head rotations at high velocity to the lesion side reduced the aVOR gain asymmetry. Seven years later, Sadeghi et al. (2018)14 showed a rebalance of the vestibular system characterized by a significant reduction of the aVOR directional preponderance in patients with chronic vestibular dysfunctions submitted to unidirectional head rotations. Rebalance resulted from a slight increased aVOR gain on the disease side and a slight decreased aVOR gain on the intact side. We have confirmed this rebalance using gaze stabilization exercises at high velocity (>200°/s) to the lesion side in acute UVH patients, and we have showed that the mechanisms involved in gaze stabilization recovery differed as a function of the time delay between onset of the vestibular disease and beginning of VR with the unidirectional rotation paradigm15. A full recovery of dynamic visual acuity was observed with early VR, due to the restoration of quiet normal aVOR on the hypofunction side, while late VR led to slight improvements only by means of compensatory saccades.
Our study was the first to suggest the existence of a critical or sensitive period after onset of the vestibular pathology, that is, an opportunity time window during which VR must be performed to obtain the best functional recovery. This crucial aspect regarding both the patients’ quality of life and the health-care costs was highlighted recently by the American Physical Therapy Association16. Among the clinical research recommendations, the first is “to examine the concept of critical period for optimal vestibular compensation through studies that examine early versus delayed interventions”. Strong supports in favor of early exposure to sensorimotor therapy had been clearly evidenced in our animal models17,18, but it is still under debate for vestibular patients. As reviewed recently by Meldrum and Jahn (2019),19 positive outcomes for the recovery of dynamic visual acuity have been found when gaze stabilization exercises are initiated in the acute stage20,21, but some other studies suggest that VR benefits can be obtained at all phases of the disease process22. Since our data in acute UVH patients suggested clearly that earlier is better for the recovery of dynamic visual acuity15, one stimulating question was to know if this critical period is observed also with different unidirectional rotation paradigms. We have therefore compared in this study two vestibular rehabilitation protocols based either on active gaze stabilization exercises or on passive whole-body rotation to the hypofunction side in different groups of UVH patients submitted to early or delayed VR.
Methods
In this paper we have compared the changes in the angular vestibulo-ocular (aVOR) gain of the semi-circular horizontal canals, as well as the directional preponderance (DP) in patients with unilateral vestibular hypofunction (UVH). This pathology (vestibular neuritis) was assessed on the basis of patients’ history and clinical examination. The inclusion criteria were in accordance with the so-called big five defined by Strupp and Magnusson (2015)23: acute onset of spinning vertigo, postural imbalance, nausea, spontaneous horizontal rotatory nystagmus beating toward the non-affected side, positive Head Impulse Test (HIT) toward the affected side (gain below 0.60, presence of overt and/or covert saccades). The aVOR gain values of the horizontal semi-circular canals were evaluated from the Synapsys software (VHIT Ulmer, Synapsys, Marseille, France) by the ratio peak eye velocity / peak head velocity, and the directional preponderance (DP) was measured by the following formula: DP = (contralateral horizontal canal gain – ipsilateral horizontal canal gain) / (contralateral horizontal canal gain + ipsilateral horizontal canal gain) X 100.
The UVH patients were submitted to two different VR protocols:
Protocol 1: One group of UVH patients (N=28) was trained to perform active gaze exercises consisting of fast head rotations towards the hypofunction side only. As for the head impulse test (HIT), they were asked to do head movements with small amplitude (10°), high velocity (200°/s) and high acceleration (around 1500°/s). When the head parameters were correctly performed, a letter was displayed on a screen during 50 ms and they were asked to recognize this optotype15. Head and eye movements were recorded with videonystagmography (VHIT Ulmer recording system, Synapsys, Marseille, France).
Protocol 2: The second group of UVH patients (N=31) was submitted to fast passive whole body rotation towards the hypofunction side only using the rotatory chair (Framiral, Grasse, France). Patients were sitting in the chair, eye closed, and submitted passively to 3 full 360° turns at high velocity (200°/s; around 1000°/s-2000°/s2). The chair was suddenly stopped at the end of the third lap; the patient was required to open his/her eyes and to fixate a stationary target located 2 meters ahead at eye level.
The two groups of UVH patients were each subdivided into three subgroups, depending on the time period between onset of the acute vertigo crisis and the beginning of VR with the gaze stabilization exercises or the rotatory chair protocol. UVH patients submitted to a VR performed as early as the first 2 weeks after onset of vestibulopathy constituted the early rehab subgroups. Patients receiving the VR between the 3rd and the 4th weeks after onset constituted the late 1 subgroups, while those with VR beginning 1 month or more after onset formed the late 2 subgroups (Table I).
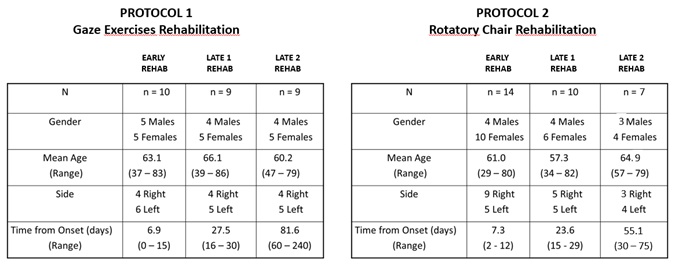
Table I: The two groups of unilateral vestibular hypofunction (UVH) patients: The table shows the number (N) of UVH patients in each of the two groups submitted either to the Protocol 1 with gaze exercises rehabilitation (N=28) or to the Protocol 2 with rotatory chair rotation (N=31). Each group was subdivided into three subgroups depending on the time delay between onset of the pathology and beginning of the vestibular rehabilitation (early, late 1 and late 2 rehab). Mean time (and range, in days) from vertigo onset and beginning of vestibular rehabilitation with one of the two protocols are shown. Gender, mean age (and range) as well as side of the hypofunction side are indicated.
The UVH patients in each of the three subgroups of each of the two populations were submitted to an equal number of training sessions, that is, two times a week for four weeks after inclusion of the patients. Measurements of aVOR Gain and DP were made just before and immediately after the end of the VR sessions by the physiotherapist during passive HIT. Five repeated passive head trusts were performed randomly to both the healthy and the hypofunction sides. It must be noticed that the two late 2 subgroups begin on the average the rehabilitation protocols when the two early subgroups had already finished their rehabilitation. Therefore, the late 2 subgroups on the first day of test had not received any specific training and can be seen as a control group without rehab. Comparing the early and late 2 subgroups allow to discern whether recovery of dynamic canal function arises from the effects of the vestibular rehabilitation protocols or from natural, spontaneous recovery.
All the patients in the present study were not under drug treatment when included and not allowed to use anti-vertigo drug treatments after inclusion. They gave written informed consent to participate. The study was conducted according to the Helsinki Declaration and the experimental protocol was approved by the local ethics committee (CCPPRB Nice).
Statistical analysis was carried out using repeated-measures analyses of variance (ANOVAs) with sub-groups (early, late 1, late 2) and parameters (aVOR gain, DP) as the between-patients factors and pre-rehab/post-rehab as the within patients factors. Moreover, given the small size of each subgroup, and the distribution of the values for each parameter in each subgroup that did not follow a normal Gaussian law, non-parametric tests were used to evaluate the effects of the two vestibular rehabilitation protocols. The Mann-Whitney U test compared the subgroups while the Wilcoxon signed rank test compared the pre- and post-rehab values. Results were considered significant at p < 0.01.
Results
The general ANOVA showed no significant differences regarding the factors age, gender, side of the horizontal canal hypofunction, and protocol. Significant differences were found between the subgroups for both the aVOR gain recorded on the hypofunction side (F (5,98) = 145.3; p < 0.001) and the DP (F (5,98) = 127.5; p < 0.001).
The figure 1 illustrates the aVOR gain values recorded on the hypofunction side before rehabilitation (Fig.1A) and after rehabilitation (Fig. 1B) with the rotatory chair protocol (upper plots) and the gaze exercises protocol (lower plots) across patients of the early (white boxplots), late 1 (grey boxplots), and late 2 (dark boxplots) subgroups. The boxplots show the median with the 1st and 3rd quartiles and whiskers indicating the minimal and maximal aVOR gain values. It must be noted that the three subgroups in each protocol showed very similar aVOR gain values before rehabilitation (p=0.64), a result strongly suggesting that the patients in the late subgroups, and particularly the late 2 subgroups, had not naturally recovered their horizontal canal function on the hypofunction side before starting the rehabilitation protocols.
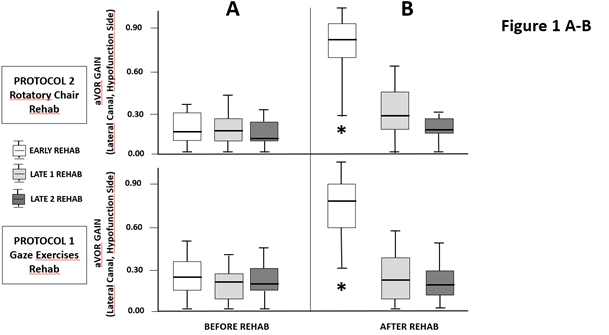
Figure 1A-B: Changes in the angular horizontal vestibulo-ocular reflex (aVOR) gain recorded on the hypofunction side: aVOR gain data from the protocol 1 (gaze exercises rehab: lower plots) and the protocol 2 (rotatory chair rehab: upper plots) measured just before the beginning of vestibular rehabilitation (A) and just after the four weeks rehabilitation period (B). The boxplots show the median with 1st and 3rd quartiles, and whiskers indicate the minimum and maximum gain values. The white boxplots for each protocol show the pre-rehab and post-rehab values recorded in the early subgroups submitted to the rehabilitation protocol (2 times a week for 4 weeks) beginning during the first two weeks after onset of the pathology. The grey and black boxplots show the gain values for the late 1 (rehab starting between the third and fourth weeks) and late 2 (rehab beginning after 1 month) subgroups, respectively. * denotes a significant increase (p<0.001) between pre-rehab and post-rehab gains.
The results showed that the changes in the aVOR gain on the hypofunction side were strongly dependent on the time delay between the onset of pathology and beginning of VR. Significant differences between pre- and post-rehabilitation were observed in the early subgroups only, with mean aVOR values of 0.29 (± 0.28) before rehab increasing to 0.69 (± 0.27) after rehab with the gaze exercises protocol (p<0.001), and of 0.23 (± 0.19) to 0.63 (± 0.34) after rehab with the rotatory chair protocol (p<0.001). The late 1 and late 2 subgroups did not show any significant changes neither with the gaze exercises protocol (0.26 ± 0.24 to 0.30 ± 0.27, and 0.20 ± 0.21 to 0.27 ± 0.30, respectively), nor with the rotatory chair protocol (0.21 ± 0.12 to 0.33 ± 0.24, and 0.16 ± 0.12 to 0.22 ± 0.11, respectively.
Similar findings were seen with the DP parameter (Fig. 2A-B; same illustration as for Fig. 1A-B). DP values represent the asymmetry between the aVOR gain recorded on the healthy and hypofunction sides before (Fig. 2A) and after (Fig. 2B) rehabilitation with the rotatory chair protocol (upper plots) and the gaze exercises protocol (lower plots) for each of the subgroups of UVH patients (early: white boxplots; late 1: grey boxplots; late 2: dark boxplots). The data showed mean values decreasing significantly in the early subgroups only, from 53.5 (± 32.1) to 27.9 (± 36.6) with the gaze exercises protocol, and from 57.8 (± 15.6) to 16.0 (± 9.8) with the rotatory chair protocol. The late 1 and late 2 subgroups did not exhibit significant changes (from 62.1 ± 38.5 to 57.5 ± 37.6 and 60.6 ± 24.3 to 56.9 ± 24.5, respectively, for the gaze exercises subgroups, and from 62.5 ± 18.7 to 44.9 ± 17.6 and 68.1 ± 18.9 to 58.8 ± 16.2, respectively, for the rotatory chair protocol).
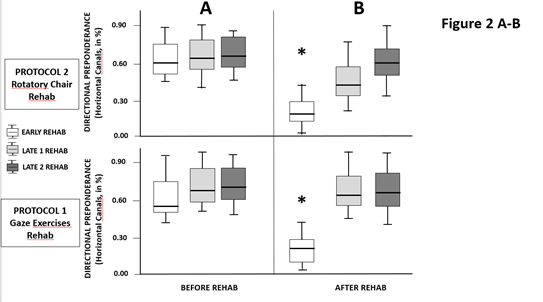
Figure 2A-B: Changes in the directional preponderance (DP) for the horizontal semicircular canals: The DP was evaluated during passive horizontal head thrust tests to both the intact and the lesion sides and expressed in percent (DP = [(contralateral canal gain − ipsilateral canal gain) â (contralateral canal gain + ipsilateral canal gain)] × 100). Same legends as in figure 1: the boxplots show the median with 1st and 3rd quartiles, and whiskers indicate the minimum and maximum DP values. The subgroups receiving early rehabilitation with either the protocol 1 or the protocol 2 are the only subgroups to exhibit significant DP reductions (p<0.001). No significant changes were observed in the late 1 and late 2 subgroups.
Interestingly, the DP decreases observed in the early subgroups resulted only from improvement of the horizontal aVOR gain recorded on the hypofunction side. No significant changes were found in the horizontal aVOR gain tested on the healthy side. For instance, the mean gain values recorded in the early subgroups were 0.82 ± 0.15 before VR to 0.86 ± 0.14 after VR for the gaze exercises protocol, and 0.86 ± 0.18 before VR to 0.87 ± 0.12 after VR for the rotatory chair protocol.
Conclusions
The present study clearly shows that 1) UVH patients can regain a near normal horizontal aVOR gain on their hypofunction side only if they are submitted to an early VR and 2) aVOR gain improvement on the hypofunction side is found with the two VR protocols using the unidirectional rotation paradigm. Improvement of the aVOR on the hypofunction side is responsible for the significant decrease of the directional preponderance, that is, the reduction of the asymmetry between the two sides since the aVOR gain recorded on the healthy side showed no changes at all.
It was believed until a recent past that the aVOR gain on the hypofunction side did not improve with passive gaze stabilization exercises24,25, but these studies included chronic UVH patients only. The sole paper to report passive aVOR gain improvement concerned patients enrolled for VR within 1 month after their vestibular neuritis attack26. We recently demonstrated that such gain improvement was limited to UVH patients submitted to gaze stabilization exercises during the early stage of the vestibular compensation process, that is, the first two post-attack weeks during which many developmental plasticity mechanisms are re-expressed15. The present results extend to UVH patients the early opportunity time window evidenced in our animal models17,18. The aVOR gain improvement seen after early VR, but not late VR, can be explained by structural processes occurring in the vestibular nuclei, like sprouting of new terminals from remaining vestibular afferents or increased number of post-synaptic receptors, two adaptive mechanisms with time constants compatible with our findings27,28, and able to reweight the vestibular input on the hypofunction side. Repair and neural reorganization at the peripheral level in the vestibular sensory epithelium cannot be excluded29 as well as learning processes implying the cerebellum30. Synaptic reorganizations could be dependent on the degree of vestibular loss on the hypofunction side, the reason why some patients in the early group with a total loss of horizontal canal function (aVOR gain = 0) did not improve as much as the others (cf Fig. 1B).
It’s therefore better to do VR early because gaze stabilization exercises performed during the critical period of neural plasticity is the sole way to restore a near normal aVOR gain and to decrease fastly the directional preponderance observed in the dynamics of the horizontal semicircular canals. When VR is performed outside this sensitive period, neural plasticity is reduced or lost and other adaptive mechanisms like the covert saccades can substitute to the abnormal aVOR4,5. However, unlike the mechanism of aVOR recovery, such a behavioral substitution process remains unable to restore a normal dynamic visual acuity15. Accordingly, to get the right vestibular diagnosis fastly and to start very early the vestibular rehabilitation are two main messages for the otorhinolaryngologists and the physiotherapists, respectively. This seems to be the only way for the patients to build an optimal functional recovery and to regain the best quality of life.
Our data also clearly show that aVOR recovery and decrease of the DP were found with the two protocols using the unidirectional rotation paradigm. It means that when performed early after onset of the pathology, both active training with gaze stabilization exercises or whole body passive rotation with the rotatory chair lead to similar improvements. A significant reduction of the DP had already been observed in both unilateral labyrinthectomized monkeys13 and UVH patients14 after training with the unidirectional rotation protocol. The authors found a DP decrease as a result of a non-significant increase of the aVOR on the hypofunction side and a non-significant decrease of the aVOR on the healthy side. The reason why the hypofunction side did not regain a normal aVOR in the above mentioned studies is very likely the long time delay between onset of the lesion and beginning of VR (2 months in the monkey, chronic and not acute patients). The only requirement to get aVOR recovery and DP decrease is therefore, to do early head/whole body rotations to the hypofunction side only. Indeed, when rotations on both sides were allowed, both DP and aVOR gain remained unchanged13.
It is reported in the literature that some UVH patients recover naturally a near normal aVOR gain with time without vestibular rehabilitation31. The proportion of UVH patients with dynamic vestibular recovery remains however low and the interesting question is to know why. Most of the UVH patients with late diagnosis and late rehabilitation report to have limited activities at home and/or to use maladaptive strategies and avoidance behaviors (do not move the head, close the eyes during head turn to the lesion side, …). The reason why they do not recover their dynamic semicircular canal function. Age and likely associated comorbidities are aggravating factors. In our study, the patients in the late 2 subgroups were not submitted to any rehabilitation before their first test, and they showed aVOR gain and DP values close to those recorded in the early subgroups at the first test. This indicates clearly that they had not recover naturally their dynamic canal function in the absence of early rehabilitation.
At the end of the XXth century, French physiotherapists had used for the first time the rotatory chair protocol to reduce the vestibular asymmetry and the DP observed during the acute phase of their UVH patients. They believed to silence the healthy side by rotating the patient to the hypofunction side by habituation, not to restore the hypofunction side by adaptation processes, but their clinical protocol was right. Similar improvements of the aVOR gain and DP were found in this study with the two protocols using either active gaze stabilization exercises or passive whole-body rotation with the rotatory chair. This clearly attests to the effectiveness of the unidirectional rotation paradigm that should be considered as a useful clinical tool in the rehabilitation program of acute vestibular loss patients.
Limitations of the study
The future should be to further evaluate this paradigm in randomized controlled trials done in larger samples of UVH patients, and in other vestibular pathologies as well. In addition, vestibular rehabilitation using the unidirectional rotation paradigm should also evaluate the effects of more intense training (increased number of rehabilitation sessions) on the aVOR gain and the DP parameter, and their meaning for the patient’s daily life. An opportunity window for the recovery of posture and balance is also a relevant question that should be tested. Finally, comparing these outcomes with the self-evaluation of the patient’s vertigo and dizziness could also contribute to a more precise evaluation of the sensitive period during which training should be done more intensively.
Conflicts of interest
None
References
- Curthoys IS, Halmagyi GM (1995) Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vest Res 5:67–107.
- Lacour M (2006) Restoration of vestibular function: basic aspects and practical advances for rehabilitation. Curr Med Res Opin 22:1651–1659. Doi.org/10.1185/03007 9906X 11569 4
- Lacour M, Barthélémy J, Borel L, Magnan J, Xerri C, Chays A, Ouaknine M (1997) Sensory strategies in human postural control before and after unilateral vestibular neurotomy. Exp Brain Res 115: 300-310.
- Herdman SJ, Schubert MC, Das VE, Tusa RJ (2003) Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg 129:819–824. Doi.org/10.1001/archo tol.129.8.819
- Schubert MC, Migliaccio AA, Clendaniel RA, Allak A, Carey JP (2008) Mechanisms of dynamic visual acuity recovery with vestibular rehabilitation. Arch Phys Med Rehabil 89:500–507. Doi.org/10.1016/j.apmr.2007.11.010
- Göttlich M, Jandl NM, Wojak JF, Sprenger A, von der Gablentz J, Münte TF, Krämer UM, Helmchen C (2014). Altered resting-rate functional connectivity in patients with chronic bilateral vestibular failure. Neuroimage Clin 12;4:488-99. Doi: 10.1016/j.nicl.2014.03.003.
- Lacour M, Helmchen C, Vidal PP (2015) Vestibular compensation: the neuro-otologist’s best friend. J Neurol. Doi. org/10.1007/s0041 5-015-7903-4
- Borel L, Lopez C, Péruch P, Lacour M (2008) Vestibular syndrome: a change in internal spatial representation. Neurophysiol Clin Clin Neurophysiol 38, 375-389.
- Young L, Bernard-Demanze L, Dumitrescu M, Magnan J, Borel L, Lacour M (2012) Postural performance of vestibular loss patients under increased postural threat. J Vest Res 22: 129-138. Doi.10.3233/VES-2012-0449.
- Hillier SL, McDonnell M (2011) Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev 2:CD005397. Doi.org/10.1002/14651 858.CD005 397. pub4
- Hillier SL, McDonnell M (2016) Is vestibular rehabilitation effective in improving dizziness and function after unilateral peripheral vestibular hypofunction? an abridged version of a Cochrane review. Eur J Phys Rehab Med 52:541–556. Doi. org/10.1002/14651 858.CD005 397.pub4
- Lacour M, Bernard-Demanze L (2014) Interaction between vestibular compensation mechanisms and vestibular rehabilitation therapy. 10 recommendations for optimal functional recovery. Front Neurol 5:285. Doi.3389/fneur.2014.00285
- Ushio M, Minor LB, Della Santina CC, Lasker DM (2011) Unidirectional rotations produce asymmetric changes in horizontal VOR gain before and after unilateral labyrinthectomy in macaques. Exp Brain Res 210:651–660. Doi.org/10.1007/ s0022 1-011-2622-2.
- Sadeghi NG, Azad BS, Rassian N, Sadeghi SG (2018) Rebalancing the vestibular system by unidirectional rotations in patients with chronic vestibular dysfunction. Front Neurol. Doi. org/10.3389/fneur .2018.01196
- Lacour M, Tardivet L, Thiry A (2020) Rehabilitation of dynamic visual acuity in patients with unilateral vestibular hypofunction: earlier is better. Eur Arch Otorhinolarynngol 277 (1) 103-113. Doi. 10.1007/s00405-019-05690-4
- Hall CD, Herdman SJ, Whitney SL, Cass SP, Clendaniel RA, Fife TD, Furman JM, Getchius TS, Goebel JA, Shepard NT, Woodhouse SN (2016) Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guidelines. J Neurol Phys Ther 40:124–155. Doi.org/10.1097/ NPT.00000 00001 20
- Lacour M, Roll JP, Appaix M (1976) Modifications and development of spinal reflexes in the alert baboon (Papio Papio) following an unilateral vestibular neurectomy. Brain Res 113: 255-269.
- Xerri C, Lacour M (1980) Role of sensorimotor activity in compensating posturo-kinetic deficits after unilateral vestibular neurectomy in the cat. Acta Otolaryngol 90: 414-424.
- Meldrum D, Jahn K. (2019) Gaze stabilisation exercises in vestibular rehabilitation: review of the evidence and recent clinical advances. J Neurol. Sep; 266(Suppl 1):11-18. doi: 10.1007/s00415-019-09459-x. Epub 2019 Aug 5.
- Enticott JC, O’leary SJ, Briggs RJS (2005) Effects of vestibulo-ocular reflex exercises on vestibular compensation after vestibular schwannoma surgery. Otol Neurotol 26: 265-269.
- Teggi R, Caldirola D, Fabiano B, Recanati P, Bussi M (2009) Rehabilitation after acute vestibular disorders. J Laryngol Otol 123: 397-402.
- Herdman SJ, Hall CD, Delaune W (2012) Variables associated with outcome in patients with unilateral vestibular hypofunction. Neurorehabilit Neural Repair 26: 151-162.
- Strupp M, Magnusson M (2015) Acute unilateral vestibulopathy. Neurol Clin 33:669–685. Doi.org/10.1016/J. ncl.2015.04.012
- Herdman SJ, Hall CD, Schubert MC, Das VE, Tusa RJ (2007) Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg 133:383–389. Doi.org/10.1001/archo tol.133.4.383.
- Tian J, Shubayev I, Demer JL (2007) Dynamic visual acuity during passive and self-generated transient head rotation in normal and unilaterally vestibulopathic humans. Exp Brain Res 142: 486-495. Doi.org/10.1007/s00221-001-0959-7.
- Kunel’s skaya NL, Naibakova EV, Guseva AL, Nikitkina YY, Chugunova MA, Manaenkova EA (2018) The compensation of the vestibulo-ocular reflex during rehabilitation of the patients presenting with vestibular neuritis. Vestn Otorinolaringol 83:27–31. Doi.org/10.17116 /otori no201 88312 7-31
- Gall C, Lynch G (1981) The regulation of axonal sprouting in the adult hippocampus: some insights from developmental studies. In: Lesion-induced neuronal plasticity in sensorimotor systems. Flohr H, Precht W (Eds). Springer-Verlag, Berlin, Heidelberg. 10.1007/978-3-462-68074-8
- Dieringer N, Precht W (1979) Mechanisms of compensation for vestibular deficits in the frog. I. Modifications of the excitatory commissural system. Exp Brain Res 36:311–328. Doi.org/10.1007/BF00238914.
- Gaboyard-Niay S, Travo C, Saleur A, Broussy A, Brugeaud A, Chabbert C (2016) Correlation between afferent rearrangements and behavioral deficits after local excitotoxic insult in the mammalian vestibule: a rat model of vertigo symptoms. Disease Models and Mechanisms ç:1181-1192. Doi:10.1242/dmm.024521.
- Ramachandran R, Lisberger SG (2008) Neural substrates for modified and unmodified pathways for learning in monkey vestibulo-ocular reflex. J Neurophysiol 100:1868–1878. Doi.org/10.1152/jn.90498 .2008
- Manzari L, Burgess AM, MacDougall HG, Curthoys IS (2011) Objective verification of full recovery of dynamic vestibular function after superior vestibular neuritis. Laryngoscope 121: 2496-2500.
