Peyronie’s disease and the role of therapeutic ultrasound: A randomized controlled trial
Joanne E. Milios*, Timothy R. Ackland, Daniel J. Green
School of Human Sciences (Exercise and Sport Science), The University of Western Australia, Crawley, Western Australia, 6009
Abstract
Peyronie’s Disease (PD) is a connective tissue disorder of the penis affecting 1 in 10 men, resulting in penile deformity and psychological distress in 50% of afflicted men. Therapeutic Ultrasound (TUS) is a non-invasive treatment that uses high frequency sound waves to stimulate tissue repair via thermal effects to increase blood flow, reduce pain and promote a proinflammatory response. TUS has been shown to be effective in case study series however no RCTs currently exist.
Methods & Materials: Forty-six men with PD were recruited into a randomised controlled study that assessed the effectiveness of TUS (n= 23 intervention, n= 20 control).
12 TUS sessions were provided over 4 weeks utilising 1.5-2.5 W/cm2, 3 MHz x 10mins/session before and after which all outcome measures were re-assessed. The control group had a 4 -week delayed entry into the intervention. Participants underwent Penile Duplex Doppler Ultrasound (PDDU) to confirm PD. as well as completing the Peyronie’s Disease Questionnaire (PDQ) and IIEF-5.
Results: Forty-three participants (59 y ± 11y, BMI=26.3, duration PD 17 months) completed the trial. PDDU outcomes indicated a Group x Time interaction (F= 4.702, p=0.036) and showed a significant main effect. Average reduction in penile curvature angle was , or 38% of total and ANOVA results show a significant main effect for time and curvature reduction (F=16.762; p < 0.001). For the IIEF-5 outcomes, results show a significant main effect for the Group x Time interaction (F=4.752, p=0.035).
Conclusions: TUS offered an effective first line, non-invasive approach to treatment for PD. Previous studies utilizing TUS on PD have relied on case study reports and this is the first RCT to be undertaken in this field.
Background/ Introduction
Peyronie’s Disease (PD) is a physically and psychologically challenging disorder that affects at least 9% of the male population and can have an impact on male self-esteem, relationships and erectile function.1-3 PD arises due to the formation of inelastic scar tissue, creating plaques in the tunica albuginea (TA) of the penis in genetically susceptible individuals or following microtrauma.4 The plaque results in palpable penile scar tissue in the flaccid state and may cause pain, penile deformities, penile curvature, hinging, narrowing and penile shortening that are generally only seen in the erect state.5
Proposed causes of PD include infection, autoimmune issues, local manifestation of fibromatosis and generalized arterial disease.6,7 The peak incidence of PD is around 55-60 years of age and is known to be associated with erectile dysfunction (ED), diabetes, obesity, hypertension, A-positive blood group, hyperlipidemia, smoking and following pelvic surgery.8 In addition, two thirds of men with PD are likely to exhibit risk factors for arterial disease and are more likely to have disease progression and worsening of ED if left untreated.4 A family history of PD in 2% of patients and an association with Dupuytren's palmar fibromatosis in 20-39%, suggest that patients may have an inherited predisposition to this disease.9,10 In addition, PD is a lesser known side-effect from treatment for prostate cancer (PCa) and is thought to occur due to injury of the cavernosal nerves and adjacent neurovascular bundles that supply erectile blood flow during radical prostatectomy (RP) and external beam radiation therapy (EBRT).11 Recent evidence confirms 16% of men undergoing RP will experience PD, at an average 13.9 months following surgery12,13 and an additional 12% of men following EBRT.14
Current treatment options for PD include penile injections, vacuum pumps, traction devices and surgery.4 As PD has an ‘acute phase’ that may last 12-18 months before stability (the ‘chronic phase’), physicians are generally reluctant to introduce treatments too early and often recommend a ‘watch and wait approach’.4,5 In the clinical setting, however, many patients are distressed by the lack of action and greatly fear the condition worsening over time. Supporting this is Mulhall’s 2006 investigations of 246 men diagnosed with PD, finding that 12% experienced a spontaneous improvement in penile curvature, 40% remained stable and 48% worsened over 12 months.15 Thus, most men with the condition will find their penile changes to be permanent, which may greatly impact on their quality of life.16 In addition, although the onset of PD might be associated with a history of buckling during sexual activity, an identified history of penile trauma is uncommon and recalled by only 10% of patients older than 40 years.8,17 A further 10% of PD presentations occur in men under 40 years, with teenagers also known to develop the condition.18 It is hypothesized that PD is commonly under-diagnosed, especially in men incapable of achieving quality erections, which may preclude the PD-associated deformity to become evident. Furthermore, embarrassment, fear of stigmatization and fear of treatment options may also mean the true incidence of PD is underestimated, with serial autopsy studies by Smith et al. confirming that 22% of men at death had PD.19
At present, there is no known cure for PD and most non-surgical treatments only offer an approximate 30% improvement.4 Hence, non-invasive treatment options to assist in the rehabilitation of PD warrant further investigation. The aim of this study was to determine the efficacy of 12 sessions of therapeutic ultrasound (TUS) as a treatment for PD so that findings may be translated into clinical practice. TUS has had a role in treating soft tissue injuries since the 1930’s, but its application to PD has been limited.20 Early case reports from the 1950’s suggested promising findings, however, no randomized controlled trials utilizing TUS have been conducted in this population.21-23 Significantly, technological advancements have become available including the use of penile duplex Doppler ultrasound (PDDU), which is considered the ‘gold standard’ approach for the diagnosis of PD.24
Materials and Methods
This study was approved by the UWA Human Research Ethics Committee (Reference: RA/4/1/8089) and all participants provided written informed consent. The trial was registered in the Australia New Zealand Clinical Trials Registry and allocated as ACTRN12617001415392.
Over a 2-year period from June 2016-2018, 46 participants with PD were enlisted from a cohort of men referred sequentially by their Urologist, GP or radiological clinic following confirmation on PDDU. Men with diabetes, taking PDE5i or other PD medications, smokers and those undergoing radiation therapy were excluded. Sequential ‘1:1’ randomization was performed as each participant attended a single high volume physiotherapy clinic following diagnosis of PD. Patients were allocated to either a ‘delayed entry’ or ‘intervention’ group based on the date of initial attendance and receipt of sequentially numbered pre-set information folders (generated by one of the authors). Relevant notes were recorded in participant medical files, which were collated over the trial duration, with results subsequently analyzed by a blinded, independent statistician.
Medical history
Prior to the trial, a full medical history pertaining to timing and probable cause of PD onset was recorded. This included the participant’s recollection of specific injury, pain, bruising or his initial awareness of a penile deformity. If no known incident was recalled, a relevant medical background such as a family history of PD, cardiovascular disease, Dupuytren’s contracture, treatment for PCa or previous pelvic surgery was ascertained. All participants were examined physically via palpation of their penis in the flaccid state. The presence of penile plaques was recorded with the location confirmed by both the participant and the PDDU report.
Intervention: therapeutic ultrasound
Over a 4-6-week period, an intervention group (n=23) attended private medical rooms to receive TUS 2-3 times per week for 10 min for 12 sessions in total. TUS dose ranged from 1.5 to 2.5 W/cm2, continuous mode, utilizing 3 MHz with a 2 cm diameter soundhead (Model: Chatoonga Intelect mobile Model 2776 SN T29173). A second ‘control’ group (n=20) had a 4-week delayed entry to the intervention, then completed the same intervention over 4-6 weeks for a total of 12 sessions. The 4-week intervention period was designed to ensure efficient methodology for participants, given the great variability in previous case study series. We did not want to prolong treatment if it was ineffective or deny the control group an opportunity for treatment if TUS proved effective.
Questionnaires were completed prior to the first session of TUS and following completion of the last therapy session. During the TUS sessions, aqueous transmission gel was applied directly to the TUS soundhead, which was then covered with a latex condom for infection control and second layer of transmission gel was applied to the external surface of the condom. Each surface was disinfected with an alcohol swab prior to and following each TUS application. Participants were then provided with the following verbal warning, “The ultrasound treatment should be maintained at a mild, comfortable warmth and at no time should there be any pain or discomfort. Please let me know if you experience any unpleasant sensations and I will cease the TUS immediately”.
Twelve TUS sessions of 10 minutes duration were then provided directly to the penile plaque with each participant positioned in supine lying with towels available for draping and participant comfort. TUS intensity commenced at 1.5 W/cm2 initially, with a gradual increase to 2.5 W/cm2 over subsequent sessions, if tolerated. Participants were advised to provide verbal feedback to reduce the TUS intensity if desired. At the conclusion of each treatment, participants were then provided with sterile wipes to remove excess transmission gel and instructed to wash their hands with disinfectant and warm soapy water.
Outcome measures
The PDDU, confirmed the size, number and position of penile plaques, and the presence of calcification.5 Measurements of the TA and plaque presentation were also outcomes, along with the angle of penile deformity and scores from the International Index of Erectile Function (IIEF-5)25 and the Peyronie’s Disease Questionnaire (PDQ).26,27 All participants were asked to provide photographic evidence of their penile curvature or deformity at the beginning and end of the trial period. The photographs were taken within one week of the first and last TUS treatment sessions. A standard clinical goniometer was applied to the photographic image to record the degree of curvature, with the axis taken from the base of the penis to the midline, in line with the urethral meatus. (Image 1).
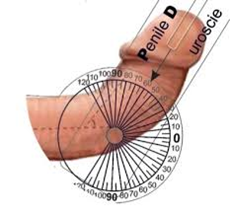
Image 1: Goniometry measurement of penile curvature in Peyronie’s disease patients.
Statistics and analysis
We could not find a previous study in which PDDU was used as an outcome measure in response to TUS in PD. We therefore based our power tests on penile curvature studies. There has also not been an RCT that utilised TUS as a treatment modality for PD. The closest exemplar that we found was the study by Ralph et al.28 who studied the impact of combined collagenase and vacuum pump therapy on penile curvature in 30 subjects with PD. Improvement in penile curvature was 23.5±9.0⸰. Our sample size included 46 participants, 43 after dropout, who were randomised to 2 groups (n=20 and 23). Given highly conservative a priori assumptions of α=0.01, a two-tailed test and a sample size of 20 per group, our study possessed >99% power to detect a similar effect size to that observed by Ralph et al.28
Outcome data were entered into SPSS (v25.0, SPSS, Chicago, IL) for analysis. T-tests for independent samples were used to check for group differences at baseline. Then a series of two-factor, repeated measures ANOVA (Group x Time) were performed and significance was accepted for all analyses at p<0.05.
Results
Of the 46 participants recruited to the study, 43 (age = 59 ± 11 y, BMI = 26.3, duration PD = 17 months) completed the study with two participants from the control group (n = 20) and one participant from the intervention group (n = 23) unable to finish due to medical illness or relocation for work. Participants were recruited over a 12-month period from April 2016 until April 2017 with follow up over a subsequent 12 months, until April 2018 when sufficient participant numbers were achieved. There were no appreciable differences in baseline characteristics between the groups (Table 1). Six participants from the intervention group and five from the control group had plaque sizes at baseline greater than 0.5 mm.
Table 1: Participant characteristics at baseline (mean ± SD) and independent t-test comparisons
|
Characteristics |
Intervention Group (n = 23) |
Control Group (n = 20) |
t |
p |
|
Age (years) |
56.8 ± 10.0 |
57.7 ± 17.0 |
0.215 |
0.831 |
|
BMI |
26.5 ± 4.7 |
26.1 ± 5.1 |
0.401 |
0.690 |
|
Duration of PD* (months) |
23 ± 33 |
16 ± 13 |
0.889 |
0.377 |
|
Angle of deformity (degrees) |
37 ± 22 |
37± 21 |
0.000 |
1.000 |
|
PDDU - size of plaque (mm) |
2.05 ± 0.66 |
1.96 ± 0.67 |
0.642 |
0.525 |
|
IIEF-5 |
16 ± 8 |
17 ± 6 |
0.458 |
0.649 |
|
PD Questionnaire |
14 ± 11 |
15 ± 10 |
0.758 |
0.310 |
* PD = Peyronie’s disease
PDDU outcomes
Results are presented in Figure 1 for intervention and control group patients from baseline (pre-treatment) to completion of 12 TUS sessions for the assessment of penile plaques (PDDU score). The ANOVA results show no significant main effect for Group (F = 0.416; p = 0.523) or Time (F = 2.35; p = 0.133), however the Group x Time interaction (F = 4.702; p = 0.036) was significant. When assessing the effectiveness of the TUS intervention, we note a difference between groups in PDDU measures, with only the intervention group having a reduction in PDDU scores.
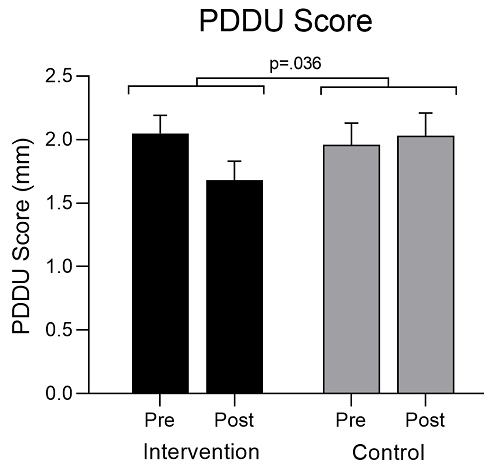
Figure 1: Changes in penile plaque size (mean ± SE) for intervention and control group patients from baseline (Pre) to completion of 12 TUS sessions (Post). This was measured by Penile Duplex Doppler Ultrasound (PDDU) and recorded as the length of the plaque (mm).
Angle of deformity
The data for angle of penile deformity are presented in Figure 2 for intervention and control groups. The ANOVA results show a significant main effect for Time (F = 16.762; p < 0.001) and the Group x Time interaction (F = 16.762; p < 0.001), but not for Group (F = 2.200; p = 0.146). There was a reduction in angle of 17ο (38%) over time for the intervention group only.
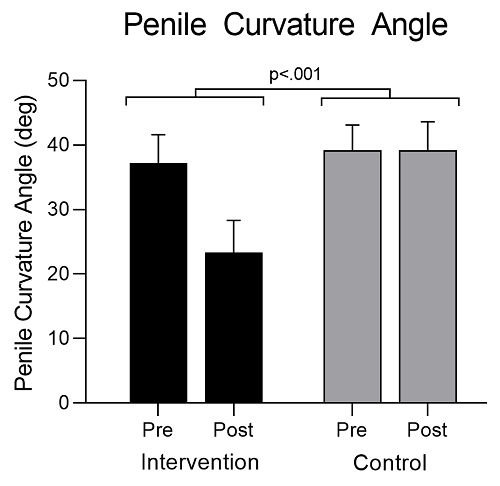
Figure 2: Changes in the angle of penile deformity (mean ± SE) for intervention and control group patients from baseline (Pre) to completion of 12 TUS sessions (Post). This was measured as the angle of deformity using photographic evidence and goniometry and recorded in degrees.
IIEF-5 scores
The data for IIEF-5 scores are presented in Figure 3 for intervention and control groups. The ANOVA results show no significant main effects for Group (F = 0.016; p = 0.900) or Time (F = 2.198; p = 0.146), but the Group x Time interaction was significant (F = 4.752; p = 0.035). Following similar scores at baseline between groups, only the intervention group improved over time.
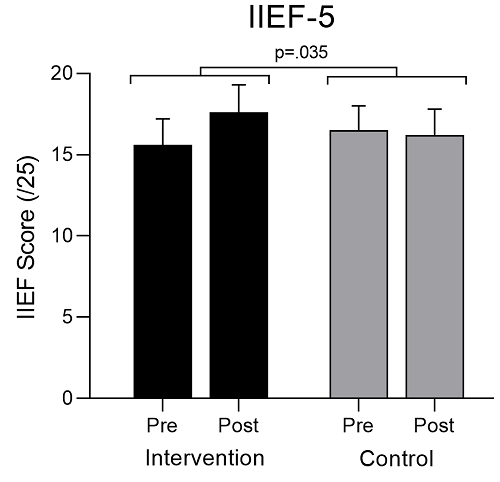
Figure 3: Changes in IIEF-5 scores (mean ± SE), indicating participant-rated erectile function from baseline (Pre) to completion of 12 TUS sessions (Post). Higher scores indicate better function (Maximum score = 25).
PDQ scores
The data are presented in Figure 4 for responses to PD via the PDQ. When data from both groups were pooled, the main effect for Time just failed to reach significance (F = 4.102; p = 0.050). The ANOVA results also revealed no significant main effect for Group (F = 0.842: p=0.365), or the Group x Time interaction (F = 1.918; p = 0.175). However, upon inspection of Figure 4, it would appear that there was a drop in PDQ scores for the intervention group, but our sample was not sufficient to provide the statistical power needed to show the impact of this intervention.
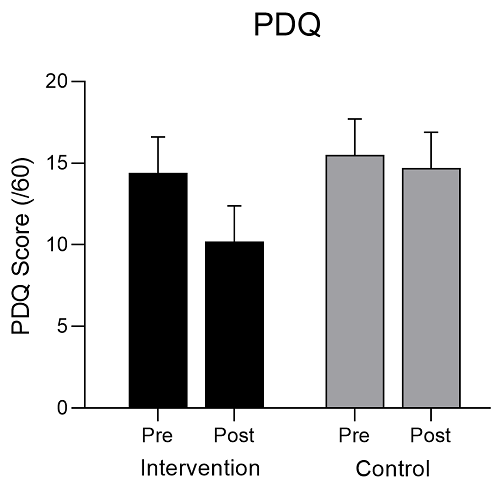
Figure 4: Changes in responses to the Peyronie’s Disease Questionnaire (PDQ) for intervention and control group patients (mean ± SE) from baseline (Pre) to completion of 12 TUS sessions (Post). Lower PDQ scores indicate better outcomes and are also reflective of the impact on participants’ partners (Maximum score = 60).
Discussion
With as many as 81% of men with PD reporting emotional difficulties, 48% clinically meaningful depression and 54% relationship difficulties, the impact of PD on individuals and their partners can be substantial.2,3,29 The challenges of PD include alterations in sexual relationships, restrictions in intimacy, socialization and stigmatization, along with deferment of relationships, which in younger patients may lead to avoidance of fathering and parenthood options.30 Improved awareness and education about the impact of PD and potential treatment options needs further development and our study aimed to address a significant gap in the availability of effective, non-invasive protocols using TUS, particularly in the acute stage of pathogenesis.
Use of TUS in the treatment of soft tissue injuries has origins that stem from the early 1930’s and uses high frequency sound waves to stimulate tissue repair.20 Frequencies used for a therapeutic effect are typically between 1.0 and 3.0 MHz. Low-frequency ultrasound waves have greater tissue penetration, but are less focused. Sound waves at 1.0 MHz frequency are absorbed at a depth of 3-5 cm and are recommended for deeper injuries and for patients with substantial subcutaneous fat.31 For more superficial lesions at a depth of 1-2 cm, 3.0 MHz frequency is recommended and, therefore, was used in this study. The dose of TUS can also be varied by altering wave amplitude and intensity, and can be used in pulsed or a continuous form. The latter form has a greater heating effect, with 40-45oC being the estimated tissue temperature rise for optimal treatment.20 Thermal effects include increased blood flow, reduction in muscle spasm, increased extensibility of collagen fibres, reduction in pain and a pro-inflammatory response.20 After reviewing previously reported protocols, a continuous TUS at 1.5-2.5 W/cm2 was selected as the treatment dose.
The PDDU scan is considered by many to be the ‘gold standard’ for assessing changes to penile tissue and determining plaque characteristics.32 This measure can demonstrate objective changes in biological tissue without the bias of subjective reporting. Of significance, the normal tissue size of the TA is 1.0-1.2 mm and this was measured both pre- and post-intervention. The average reduction in TA was 0.37 mm, indicating a treatment effect that was not observed in the control group patients. Reductions in plaque size were mirrored by participant reports of reduced size and hardness of plaques, improved malleability of the penis and satisfactory improvements in curvature and physical deformities. Importantly, participants displaying calcified plaques >0.5 mm at baseline were less likely to respond to TUS, however, several individuals with ‘focal’ calcified plaque formation of <0.5 mm had complete resolution of this presentation. This is an important consideration for the selection of patients for future treatment.
Improvement in the angle of deformity is possibly the most important outcome for patients, given the potential impacts on self-esteem, sexual function and relationships. The intervention group reported an average 17ο reduction in curvature, however, several participants who had other penile deformities, such as indentations and hour-glass formations, also observed improvements in penis shape and appearance. In these individual cases, PDQ scores were greatly improved, reflecting a positive psychological outcome. In particular, all participants reported a resolution of pain, a significant QOL outcome, having positive translation to the re-engagement or improvement of sexual activity and frequency.
Examination of erectile function via the IIEF-5 questionnaire, which is often closely related to PD, was another outcome measure. The intervention group displayed improvement in scores, indicating a treatment effect when compared to the control group. The average improvement in IIEF-5 scores was three points which is clinically relevant, representing a shift in category from ‘mild to moderate ED’ to ‘mild ED’.26
The PDQ score provided an opportunity to assess the psychological impact of PD on both the individual and his relationships. Although our analysis was not able to show significance (p = 0.05; trend only), individuals who were most improved in other measures, showed similar improvements in PDQ scores over time. The PDQ was seen as an important measure in the clinical setting in this study and has been recommended by others as being a valid assessment tool.28
Since the 1950’s there have been some experimental studies utilizing TUS for PD, however, given the lack of controlled trials and employment of patient reported outcomes in previous studies, TUS has not evolved as a mainstream treatment option. Dugois’s 1951 publication was the first to report the treatment of PD with TUS and, in a series of 20 cases, he was able to recommend a minimum of 20 sessions per patient for the successful treatment of PD.21 However, the data collected was based on observation over 8 years of clinical experience. In addition, an active ingredient α-chymotrypsin, was also utilized and the efficacy of TUS alone could not be ascertained. A smaller investigation by Heslop et al. in 1967 reported on 9 patients who received TUS for PD. All had a reduction in pain and 4 reported complete resolution of palpable plaques.22 Similarly, Liakhovitskii applied TUS to 67 patients and noted a decrease in penile pain in almost all patients after 3-5 sessions, and the absence or marked decrease in 52 patients after 20-25 TUS applications.33 Improvements in penile curvature and palpable plaque size were also reported, but to a lesser degree. In both series no adverse side effects were reported.
More recent research to assess the impact of TUS on PD involved a case series published by Miller et al. in 1983, which included a cohort of 30 men receiving TUS over a 5-year period from 1977 to 1982.34 Participants received a variable number of treatments, designed around 2 week courses of daily TUS, with a range of 1-6 courses undertaken. A standard dose of 1.5 W/cm2 for 5 minutes was the prescribed treatment protocol, with participants self-regulating the frequency of courses over the 5-year period of assessment. In addition, a hydrocortisone ointment was used as the conducting agent for each session, based on evidence produced by Griffin et al. in earlier investigations advocating its benefits in combination with TUS for reducing inflammation.35 Of the 25 men who completed treatment, 19 reported improvement with 16 reporting reduced plaque size, 4 reporting resolution of plaque, 9 nine reporting reduced pain and 4 reporting reduced penile deviation. Miller concluded that treatment benefit was most effective with earlier presentations of PD, although a positive effect was still seen in cases who presented after 1 year. Given these observations, TUS was recommended as a non-invasive treatment option, which was repeatable and led to softer, more flexible plaques that were less deforming, less prominent and less painful.34
The most recent publication exploring the use of TUS in PD by Kos et al., explored the case of a 49-year-old patient who received 90 TUS sessions over an 18-month period (protocols - 2.0 W/cm2 for 8 minutes per session). Assessment methods included the IIEF-5, the Visual Analogue Scale and measurement of plaque volume via PDDU, with outcomes showing significant improvement in all parameters. Interestingly, the particular medical facility that produced this paper, the University Medical Centre of Ljubljana in Slovenia, reported that patients with PD had being treated with TUS at their institution for almost 30 years, with empirically good results, despite the lack of objectivity in outcome measures.36
Hence, the variability in treatment frequency, however, makes the comparison of results between studies challenging, despite the generally positive outcomes from each study.
Participants in our study (n=43) derived PD from a range of aetiologies with a sub-group of 10 men being treated for PCa (n=8 following surgery, n=2 following EBRT). PD resulting from trauma occurred in three cases, pelvic surgery precipitated PD in two participants, and another two had a confirmed diagnosis of chronic pelvic pain syndrome (CPPS) which has been associated with sexual dysfunction and reduced blood flow due to hypertonic pelvic floor muscles.37 One patient presented with the triple combination of PD, Dupuytren’s contracture and Lederhosen Disease (hard lumps of connective tissue in the plantar aspect of the foot), and another six participants in the total cohort had confirmed Dupuytren’s contracture. One patient reported PD following a combination of chemotherapy and EBRT for pelvic cancer, while all other cases had no specific trigger. This represents a total of 58% of the cohort having an established link to identified causes, with the remaining 42% not able to pinpoint a triggering event or co-existing pathology.
As the first randomized controlled trial to assess the impact of TUS on PD, a treatment effect was confirmed in the intervention group compared to the control group. The TUS protocols we used afforded clinically relevant reductions in plaques of the TA, penile curvature and pain, with improvement in IIEF-5 scores. Similar to findings by Miller, participants in the present study with earlier presentations of PD generally responded better than more long-term cases.34 A younger patient (33 years of age with a 13-year history of PD), however, achieved excellent score reductions in PDDU (from 2.4 to 1.2 mm) and penile curvature (from 40 to 10 degrees). Furthermore, in our series, individuals who did not respond to TUS had confirmed calcification of plaques larger than 0.5 mm. Those with smaller areas of foci calcifications typically responded to TUS and the problem resolved.
Given the large range of TUS sessions/participant (3-25) reported in the aforementioned studies, it is encouraging to note that the 12 TUS sessions undertaken in our study were able to produce significant, positive outcomes. This also identifies a possible limitation in our results. Had this number been increased to 20-25 sessions, the results may have been more impactful. However, our aim was to provide a clinical treatment that could be reviewed after 12 sessions, with the time and financial cost to patients minimized. This baseline provides clinicians with an option to continue therapy if only partial TA reduction was noted, or to cease TUS if no improvement or a complete resolution of PD is noted. As found in our cohort, the presence of calcified plaques >0.5 mm may also assist patient selection, as TUS appears to be ineffective for these patients.
The overall outcomes of our study including resolution of penile pain, cessation of PD progression, and reduction in plaque size and penile curvature are important clinical findings in the potential future management of PD. Early diagnosis via broader education and awareness of early intervention strategies such as TUS could reduce the manifestation of the more chronic, complex presentations including severe calcification. By this stage, surgery is recognized as the only curative option for treatment, but may also lead to worsening of erectile function, and complications including pain, infection, more costly intervention, and enhanced psychological distress. Physiotherapy approaches that provide early intervention strategies prior to the calcification of PD and can be immediately delivered to patients are recommended from our results.
Conclusion
In conclusion, we present a non-invasive treatment option for PD patients, utilizing experience gained from previously published clinical case series. TUS is particularly useful when the calcification of plaques, as confirmed by PDDU scans, has been eliminated. Given the potential psychological distress for men with PD, our findings indicate that treatment with TUS, especially in the early phases of PD, reduced penile pain, improved penile deformity and increased erectile function. TUS represents an effective, inexpensive treatment option for men suffering with this difficult to treat affliction, causes no harm, can be provided by any qualified physiotherapist and may be immediately translated to effective, clinical practice.
Acknowledgements
I would like to thank Dr Alar Kaard and Ultrasonographer Chris Bevan from SKG Radiology Hollywood, Western Australia for their assistance with Penile Duplex Doppler scans and Dr Stephen Adams for assistance with recruitment and support.
Declaration of Interest
Competing interests
The authors declare that they have no competing interests and do not have any financial or personal relationships that could bias this work.
Ethics, consent and permissions
This study was approved by the UWA Human Research Ethics Committee (Reference: RA/4/1/8089) and all participants provided written informed consent.
Trial Registration
The trial was registered in the Australia New Zealand Clinical Trials Registry and allocated as ACTRN12617001415392 and retrospectively registered.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public,commercial or not-for-profit-sectors.
Conflict of Interest
There are no potential conflicts of interest in the submitted research and all participants provided informed consent prior to inclusion in the study. This research involved human participants, however none were exposed to any potential harm, as all assessments made were either by questionnaire or non-invasive real time ultrasound approaches.
Author’s Contribution
All authors read and approved the final manuscript
JM- was involved in the project and protocol development, data collection and had a minor role in data analysis.
DJG – was involved in the protocol and project development, data management and data analysis.
TA- was involved in the data management and data analysis and oversaw the project development.
References
- Mulhall JP, Creech DC, Boorjian SA, et al. Subjective and objective analysis of the prevelance of peyronies disease in a population of men presenting for prostate cancer screeening. J. Urol. 2004;171(6):2350-2353.
- Nelson CJ, Mulhall JP. Psychological impact of Peyronie's disease: A review. J Sex Med. 2013;10:653-660.
- Farrell MR, Corder CJ, Levine L. Peyronie's disease among men who have sex with men: characteristics, treatment and psychosocial factors. J Sex Med. 2013;10:2077-2083.
- Chung E, Ralph D, Kagioglu A, et al. Evidence-based management guidelines on Peyronie's Disease. J Sex Med. 2016;13(6):905-923.
- Kalokairinou K, Konstantinidis C, Domazou M, Kalogeropoulos T, Kosmidis P, A. G. US imaging in Peyronie's disease. J Clin Imaging Sci. 2012;2(63). Accessed 22 Oct 2018.
- Ralph DJ, Schwartz G, Moore W, Pryor JP, Ebringer A, GF. B. The genetic and bacteriological aspects of Peyronie's disease. J Urol. 1997;157:291-294.
- Ralph D, Gonzalez-Cadavid N, Mirone V, et al. The management of peyronies disease: evidence-based 2010 guidelines. J Sex Med. 2010;7:2359-2374.
- Love C, Katz DJ, Chung E, Shoshany O. Peyronie's Disease- watch out for the bend. Urology. 2017;46(9):655-659.
- Chilton CP, Castle WM, Westwood CA, JP. P. Factors associated in the etiology of Peyronie's disease. Br J Urol. 1982;54:748-750.
- Bjekic MD, Vlajinac HD, Sipetic SB, Marinkovic J. Risk factors for Peyronie's Disease: A case-control study BJU Int. 2006;97(3):570-574.
- Dean RC, Lue T. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin Nth Amer. 2005;32(4):379-v.
- Teloken PE, JP. M. Erectile Function Following Prostate Cancer Treatment: Factors Predicting recovery. Sexual Medical Review 2013;1:91-103.
- Tal R, Heck M, Teloken PE, Siegrest T, Nelson CJ, Mulhall JP. Peyronie's Disease Following Radical Prostatectomy: Incidence and predictors. J Sex Med. 2010;7:1254-1261.
- Frey A, Pedersen C, Lindberg H, Bisbjerg R, Sønksen J, Fode M. Prevalence and Predicting Factors for Commonly Neglected Sexual Side Effects to External-Beam Radiation Therapy for Prostate Cancer. J Sex Med. 2017;14(4):558-565.
- Mulhall JP, Schiff J, Guhring P. An analysis of the natural progression of Peyronie's disease. J Urol. 2006;175:2115-2118.
- Nelson CJ, Diblasio C, Kendirci M, Hellstrom W, Guhring P, Mulhall JP. The chronology of depression and distress in men with Peyronie's disease. J Sex Med. 2008;5:2179-2184.
- Kadioglu A, Tefekli A, Erol B, Oktar T, Tunc M, Tellaloglu S. A retrospective review of 307 men with Peyronie's Disease. J Urol. 2002;168(3):1075-1079.
- Tal R, Hall MS, Alex B, Choi J. Peyronie's Disease in teenagers. J Sex Med. 2012;9:302-308.
- Smith B. Subclinical Peyronie’s disease. . Am J Clin Pathol 1969;52:385-390.
- Speed C. Therapeutic ultrasound in soft tissue lesions. Rheumatology. 2001;40:1331-1336.
- Dugois P. The action of ultrasonics on Peyronie's disease ; accelerated by a-chymotrypsin. Lyon Med. 1951;93(218).
- Heslop RW, Oakland DJ, Maddox B. Ultrasonic therapy in Peyronie's disease. Br J Urol. 1967;35:415.
- Frank IN, Scott W. The ultrasonic treatment of Peyronie's Disease. The J o Urol. 1971;106:883-887.
- Yoganandan N, Pintar F, Humm J, Rudd R. Injuries in Full-Scale Vehicle Side Impact Moving Deformable Barrier and Pole Tests Using Postmortem Human Subjects. Traffic injury prevention. 2015;16 Suppl 2:S224-230.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
- Hellstrom W, Feldman R, Rosen RC, Smith T, Kaufman G, Tursi J. Bother and distress associated with Peyronie's Disease: validation of the Peyronie's disease questionaire. J Urol. 2013;190(2):627-634.
- Coyne KS, Currie BM TC, Smith T. The tes-retest reliability of the Peyronie's Disease questionaire. J sex Med. 2015;12(2):543-548.
- Ralph DJ, Raheem A, Liu G. Treatment of Peyronie's Disease with Collagenase Clostridium Histolyticum and Vacuum Therapy: A Randomized, Open-Label Pilot Study J Sex Med 2017;14(11):1430-1437.
- Davis SN, Ferrar S, Sadikaj G, Gerard M, Binik YM, Carrier S. Female partners of men with Peyronie's disease have impaired sexual function, satisfaction and mood while degree of sexual interference is associated with worse outcomes. J Sex Med. 2016;13(7):1095-1103.
- Danelson KA, Kemper AR, Mason MJ, et al. Comparison of ATD to PMHS Response in the Under-Body Blast Environment. Stapp car crash journal. 2015;59:445-520.
- Gann N. Ultrasound: current concepts. Clin Manag. 1991;11:64-69.
- Hussein AA, Alwaal A, Lue T. All about Peyronie's disease. Asian Journal of Andrology. 2015;2(2):70-78.
- Liakhovitski N. Experience in the use of ultrasonics in the therapy of plastic induration of the penis. Urologia. 1960;25(64).
- Miller HC, Ardizzone J. Peyronie Disease treated with ultrasound and hydrocortisone. Urology. 1983;21(6):584-585.
- Griffin JE, Echternach JL, Price RE, Touchstone J. Patients treated with ultrasonic hydrocortisone and with ultrasound alone. Phys Ther. 1967;47:595.
- Kos N, Brcar M. Is therapeutic ultrasound efficient in treating Peyronie's disease? - Case Report. IOSR J o Dental & Med Sci 2016:15(10):63-66.
- Cohen D, Gonzalez J, Goldstein I. The role of pelvic floor muscles in male sexual dysfunction and pelvic pain. Sexual medicine reviews. 2016;4(1):53-62.
